- Title
-
Artery-vein specification in the zebrafish trunk is pre-patterned by heterogeneous Notch activity and balanced by flow-mediated fine tuning
- Authors
- Geudens, I., Coxam, B., Alt, S., Gebala, V., Vion, A.C., Meier, K., Rosa, A., Gerhardt, H.
- Source
- Full text @ Development
|
|
|
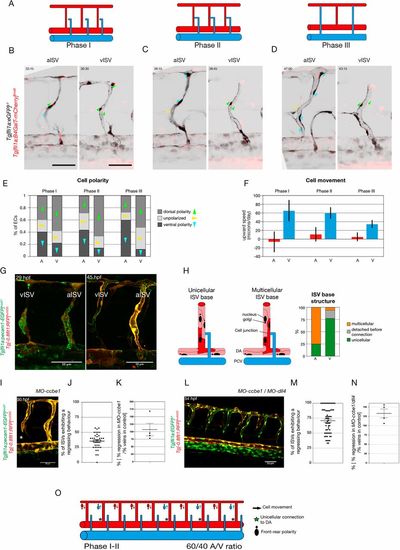
Primary ISVs are specified into aISVs and vISVs prior to connection by secondary sprouts originating from the PCV. (A) Schematic representation of the three phases of primary ISV remodelling: phase I, before secondary sprout connection to the primary ISV; phase II, when a lumenised connection is formed between the secondary sprout and the primary ISV; phase III, when the three-way connection resolves into aISV or vISV. (B-D) Stills from time-lapse movies (see Movies 4 and 5) of EC polarity in aISVs and vISVs of Tg[fli1a:GFP]y1;Tg[fli1a:B4GalT-mCherry]bns9 embryos during the three different phases: (I) before secondary sprout connection, (II) during three-way connection and (III) after resolution. Arrowheads indicate the angle from the centre of the nucleus to the centre of the Golgi complex: green indicate dorsal polarity; blue indicate ventral polarity; yellow indicate unpolarised ECs. (E) Quantification of EC polarity in aISVs (n=7 aISVs, 16 cells) and vISVs (n=8 vISVs, 17 cells) of Tg[fli1a:GFP]y1;Tg[fli1a:B4GalT-mCherry]bns9 embryos during the three different phases: (I) 2.5 h before secondary sprout connection; (II) during three-way connection; and (III) 2.5 h after resolution. (F) Quantification of EC upward speed (in microns/day) in aISVs (n= 12 aISVs, 67 cells) and vISVs (n=13 vISVs, 103 cells) during the three different phases: (I) 1 h before secondary sprout connection; (II) during three-way connection; and (III) 1 h after resolution. (G) Stills from time-lapse movie (Movie 6) of a Tg[fli1a:pecam1-EGFP]ncv27;Tg[-0.8flt1:RFP]hu5333 embryo showing ISV remodelling into an arterial and a venous intersegmental vessel (aISV and vISV) at 29 and 45 hpf. (H) Quantification of the cellular structure at the base of the primary ISV at the inception of phase II. Detection or absence of GFP expression is used to characterise the nature of the connection (unicellular or multicellular) (n=34 embryos, 12 aISVs, 40 vISVs). (I) Still from a time lapse movie (see Movie 7) of a Tg[fli1a:pecam1-EGFP]ncv27; Tg[-0.8flt1:RFP]hu5333 5 ng MO-ccbe1 embryo showing ISV regression in the absence of secondary sprouting. (J) Quantification of the percentage of primary ISVs exhibiting a regression behaviour (full disconnection from the DA, thin membrane connection to the DA, lumen collapse and reconnection, and cell death at the base of the primary ISV; see Fig. S3) (n=4 experiments, 37 morphants, 241 morphant vessels). (K) Quantification of percentage of primary ISVs exhibiting a regression behaviour in ccbe1 morphants compared with the percentage of veins in control clutch mates (n=37 morphants, n=29 wild-type controls). (L) Stills from time-lapse movie (Movie 8) of a Tg[fli1a:EGFP]y1;Tg[-0.8flt1:RFP]hu5333 5 ng MO-ccbe1/10 ng MO-dll4 embryo showing ISV regression in the absence of secondary sprouting. (M) Quantification of percentage of primary ISV exhibiting a regression behaviour (full disconnection from the DA, thin membrane connection to the DA, lumen collapse and reconnection, and cell death at the base of the primary ISV; see Fig. S2) (n=7 experiments, 62 morphants, 531 morphant vessels). (N) Quantification of percentage of primary ISVs exhibiting a regression behaviour in MO-ccbe1(5 ng)/MO-dll4 (10 ng) double morphants compared with the percentage of veins in control clutch mates (n=62 morphants, n=17 wild-type controls). (O) Schematic representation of ISV specification prior to and at the inception of the three-way connection, quantifiable through EC polarity, upward movement speed and cellular structure at the connection to the dorsal aorta. A, aISV; V, vISV. Scale bars: 50 μm. In J,K,M,N, data are mean± s.e.m. with individual data points indicated. |
|
|
|
|
|
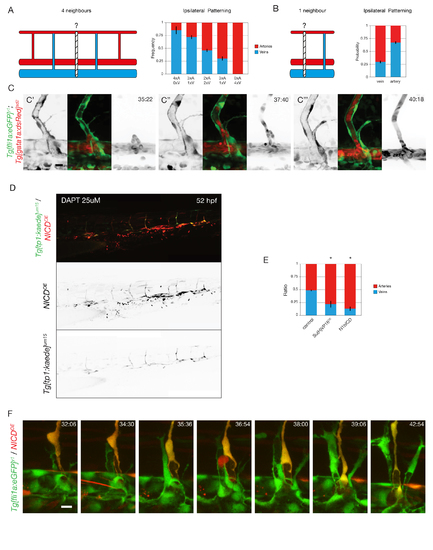
A) Ipsilateral neighborhood analysis of vessel identity with 4 neighbors in 6 dpf WT embryos (N=3 experiments, 74 embryos, 888 ISVs). B) Ipsilateral neighborhood analysis of vessel identity with 1 neighbor in 6 dpf WT embryos (N=3 experiments, 74 embryos, 1332 ISVs). C) Stills from time-lapse movie (Supplementary Movie S2) in Tg[fli1a:GFP]y1;Tg[gata1a:DsRed]sd2 labeling ECs in green and blood cells in red showing formation of a transient perfused three-way connection (C’-C”) as circulating blood cells can be observed in the DA-ISV-secondary sprout-PCV shunt. In panel C”’ it is clear that the ISV-PCV connection is disconnected again and the secondary sprout takes part in lymphatic development, whereas strong dorsal flow is established in the arterial ISV. D) Tg[tp1-MmHbb:kaede]um15 embryo mosaically expressing a pT2Fli1ep-zN1aICD-basflimCherry construct (NICDOE) at 52 hpf. Endogenous Notch activity was blocked by treatment with 25μM DAPT from 24 till 52 hpf in order to observe Notch activation by NICD overexpression. E) Quantification of the ratio of arterial and venous ISVs containing cells overexpressing Su(H)VP16, a constitutively active variant of the Su(H) transcription factor (Su(H)VP16OE) (N=3 experiments, 29 embryos) or mosaically overexpressing Notch-1bICD (N1bICDOE), the intracellular domain of Notch1b, the paralogue of Notch1a (N=2 experiments, 43 embryos). (*P=0.0001) F) Stills from time-lapse movie (Supplementary Movie S3) in Tg[fli1a:GFP]y1 embryos mosaically overexpressing a pTol2-zN1aICD-basfli-mCherry construct showing formation of a transient perfused three-way connection between a wild-type secondary sprout and a NICD overexpressing (NICDOE) primary ISV, indicating that Notch activation does not prevent interaction between primary ISVs and secondary sprouts. |