- Title
-
Zebrafish as an Emerging Model for Osteoporosis: A Primary Testing Platform for Screening New Osteo-Active Compounds
- Authors
- Bergen, D.J.M., Kague, E., Hammond, C.L.
- Source
- Full text @ Front Endocrinol (Lausanne)
|
Rapid and efficient mutagenesis using CRISPR/Cas9 genome editing in zebrafish. |
|
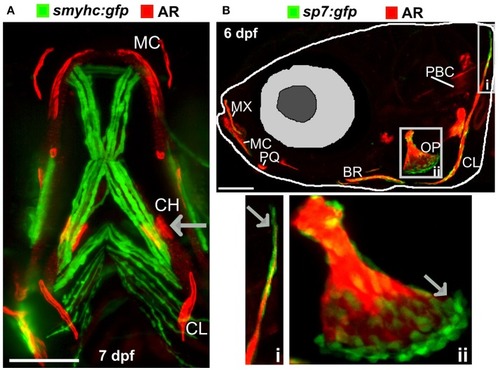
Ossified elements in the cranial region during early development. |
|
Examples of visualization and quantification of mineralized bone in zebrafish. |
|
Fin regeneration and fracture assay to visualize and quantify live bone formation and repair. |
|
Zebrafish elasmoid scale structure and bone cell types. |
|
Schematic representation to show how osteoblast activity can be quantified from scales. Scales from |
|
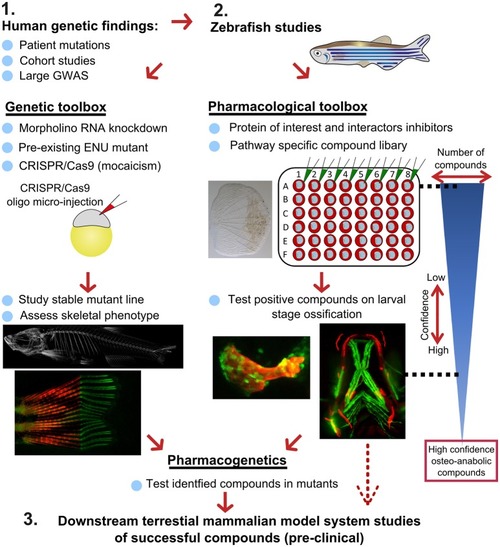
Proposed pipeline using zebrafish as a primary testing platform to address bottleneck for fast and affordable translation of human genetic findings. Two experimental arms using the genetic and pharmacological toolboxes allow simultaneous drug target validation. The blue reversed triangle depicts the reduction in number of putative osteo-anabolic compounds (along with an increase in confidence) when testing the compounds using the skeletal assays available. |