- Title
-
Heat shock modulates neutrophil motility in zebrafish
- Authors
- Lam, P.Y., Harvie, E.A., and Huttenlocher, A.
- Source
- Full text @ PLoS One
|
Heat shock effects on neutrophil recruitment. (A) Schematic illustration of the experimental setup for otic vesicle infection shown in C. (B) Schematic illustration of the experimental setup for tail fin wounding shown in E. The red rectangle indicates heat shock at 38-39°C for 1 hour. Grey circles indicate the time point for neutrophil counting. (C) Quantification of neutrophils at the otic vesicle (yellow dotted line) in control (cntl) and heat shocked (HS) larvae at 2 hours post infection (2 hpi). Larvae were injected with 100 CFU of Streptococcus iniae (S.i) into the otic vesicle (ear) at 3 days post fertilization (dpf). HS larvae showed decreased neutrophil recruitment compared with controls. **P<0.01 (two-tailed, unpaired t-test). (Right panel) Representative images of Sudan Black-stained larvae. Lateral view of the head of larvae at 2 hpi. (D) Survival of control (cntl) and heat shocked (HS) larvae infected with S.i. in the ear over time. Control and HS larvae showed a similar survival rate. dpi; days post infection. (E) Quantification of neutrophils at wounds in control (cntl) and heat shocked (HS) larvae at 1 and 4 hour post wounding (hpw). HS larvae showed fewer neutrophils at wounds at 4 hpw compared with controls. ****P<0.0001; ns, not significant (two-tailed, unpaired t-test). (Right panel) Representative images of Sudan Black-stained larvae. Lateral view of the tail fin of larvae at 3 dpf. (F) Quantification of neutrophils in whole larvae for control (cntl), heat shocked (HS) and 3 hours post heat shocked (3 hpHS) larvae. (Right panel) Representative images of Sudan Black-stained larvae. Data are representative of at least three experiments. PHENOTYPE:
|
|
Heat shock induces transient changes in neutrophil random motility and sustained effects on recruitment to wounds. (A) (Top) Schematic illustration of the experimental setup for quantification of neutrophil speed. The red rectangles indicate heat shock at 38-39°C for 1 hour. Arrows indicate the time point for neutrophil live imaging in the same larva before heat shock (cntl), right after heat shock (HS) and at 3 hours post heat shock (3 hpHS). (Bottom) Scatter plot showing the mean speed of Tg(mpx:dendra2) neutrophils at time points indicated. Neutrophils were tracked in 3 dimensions (3D) using Image J software and the MTrackJ plugin. HS larvae showed increased neutrophil speed compared with controls. At 3 hpHS, the speed of neutrophils went back to control levels. **P<0.01; ns, not significant (one way ANOVA with Dunn’s multiple comparison test). (B) (Top) Schematic illustration of the experimental setup for tail fin wounding. Larvae were wounded without heat shock (cntl), right after heat shock (HS) or at 3 hours post heat shock (3 hpHS). Larvae were then fixed at 4 hours post wounding (hpw) for quantification of neutrophils (grey circles). (Bottom) Quantification of neutrophils at tail fin wounds at 4 hpw. The effect of HS on neutrophil wound response persisted even when the wound was induced at 3 hpHS. ****P<0.0001 (one way ANOVA with Dunn’s multiple comparison test). Data are representative of at least three experiments. PHENOTYPE:
|
|
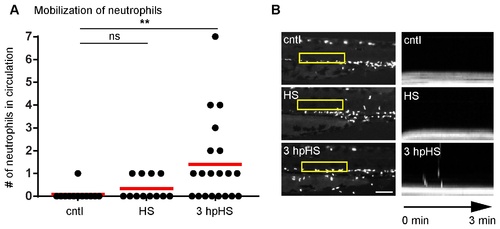
Heat shock induces neutrophil mobilization. (A) Quantification of circulating neutrophils in larvae at 3 dpf. There was an increase in circulating neutrophils at 3 hpHS. **P<0.01; ns, not significant (one way ANOVA with Dunn’s multiple comparison test). (B) (Left panel) Yellow box indicates area where kymograph was generated. Fluorescent signal in boxed region was stacked vertically into a one-dimensional line at each time point. Scale bar = 100 μm. (Right panel) Kymograph of 3 minute movies with a 3 second interval indicating the presence or absence of circulating neutrophils is shown. Data are representative of at least three experiments. PHENOTYPE:
|