- Title
-
Sox9-related signaling controls zebrafish juvenile ovary-testis transformation
- Authors
- Sun, D., Zhang, Y., Wang, C., Hua, X., Zhang, X.A., and Yan, J.
- Source
- Full text @ Cell Death Dis.
|
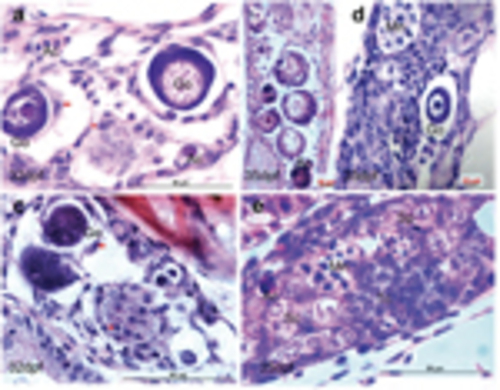
Gonadal development and differentiation. Indifferent gonad (a), only primordial germ cells (PGCs) are found in the gonad at 5 d.p.f. PGCs contain a large nucleus and possess a distinct nuclear membrane, several big nucleoli, and a network of fine chromatin filaments distributed throughout the karyoplasm. Along the inner side of the nuclear membrane, deeply staining granulosa material is seen. The weakly stained cytoplasm accounts for only a small fraction of the cell volume. At 7 d.p.f., PGCs differentiate into gonocytes (Gc). At 11 d.p.f., more Gcs and meiotic gonocytes with condensed chromatin (Cn) are seen, in addition to PGCs. At 17 d.p.f., early perinucleolar oocytes (EPOs) appear. During juvenile gonadal differentiation toward the ovary (b), more meiotic germ cells are seen (23 d.p.f.), showing densely packed oocytes (27 d.p.f.) and tight connections between late perinucleolar oocytes (LPOs) at 35 d.p.f. In the presumptive testis (c), stromal cells increase and oocyte degenerations appear as acid staining cells (Ac). Sc, spermatogonia cyst. Scale bar=20μm |
|
Morphological alterations from ovarian follicle to testis cord. (a) Active stromal cells surround the giant oocytes (Go) and form degenerative compartment (a). Within the degenerative compartment, follicle cells dissociate from the oocytes (b) and acquire mesenchymal morphology (arrow, a). (c) Degeneration of perinucleolar oocytes (primordial follicles) with marked vacuolation and survival cells from the oocyte decomposition (arrowhead). (d) Coexistence of oocyte degeneration and multinucleated giant cells (Mn) that present many peripheral nuclei and annular chromatin. (e) Regeneration of testis cord tissues. Ac, acidic cells with red coloration; Sc, spermatogenic cysts; Sp, spermatogonia; Tc, testis cord-like structure. Scale bars= 20μm (b, and d) and 50μm (a, c and e) |
|
Comparative analysis of the expression pattern of multiple sex determinant genes between transforming and nontransforming gonads at 35 d.p.f. In the nontransforming gonads, vasa (a) was highly expressed whereas sox9a (b) expression was restricted in the two sides of the gonadal somatic cells. The transforming gonads revealed distinct expression patterns for vasa (c), sox9a (d), amh (e), cyp19a1a (f), and dzip1 (g and h). amh, cyp19a, and sox9a were expressed in somatic cells (supporting cells) located in the gonadal matrix and surrounding the oocytes. dzip1 was highly expressed in both spermatogonia and supporting cells. (e and f) Two adjacent sections with low levels of cyp19a1a and high levels of amh expressed with partial overlap in the same follicles (asterisk). Abnormally expressing aggregates in degenerative compartment (Dc) are indicated by the arrow. TUNEL staining assay showed the larger TUNEL-positive cells were consistent with degenerative oocytes (i). Hoechst staining (j) was used to count total number of gonadal cells. Go, giant oocyte; Mn, multinucleated giant cells; PO, perinucleolar oocyte. Scale bars=20μm |
|
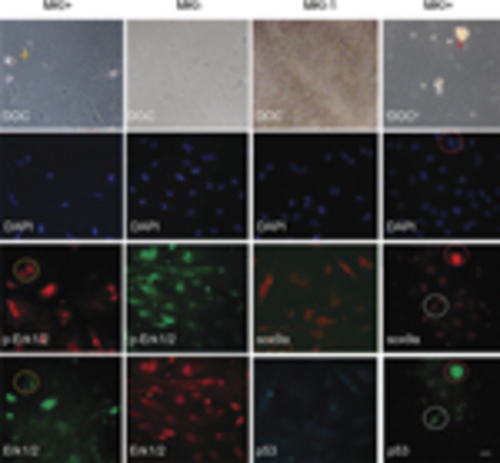
Involvement of MAPK-sox9a and p53 pathways in morphological alterations of cultured gonadal tissue. Ovary tissues were sliced and cultured in vitro. After 2–3 days, MKI or DMSO control were added and tissues were incubated for 48h. The cultures were costained with Erk1/2 and p-Erk1/2 antibodies (left two columns) or costained with sox9a and p53 antibodies (right two columns). DAPI staining and DOC were seen. Apoptotic or degenerating cells (arrow) and related signal staining (dotted circle) are shown. Scale bars=20μm |
|
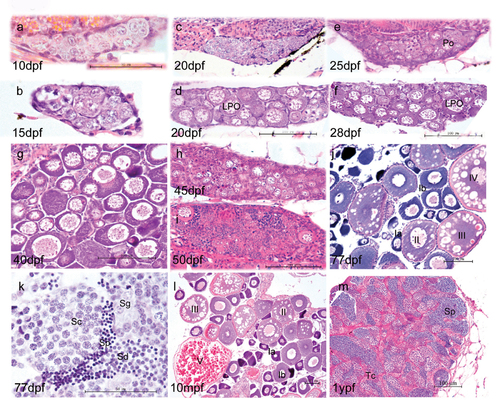
Morphological development of zebrafish gonads. At 10 dpf and 15 dpf (a, b), the majority of gonad cells are different types of germ cells, surrounded by basophilic somatic cells. In the subsequent juvenile period, some gonads show fast development with more meiotic germ cells and more condensed late perinucleolar oocytes (LPO) (d, f). The other gonads show more stromal cells and fewer LPO (c, e). By 40 dpf, most fish have undergone sex differentiation (g), although a few individuals still undergo ovary-testis transformation at 45 dpf (h) and 50 dpf (i). At 77 dpf, fish complete sex differentiation (j, k). j and l show the mature ovary at 77 dpf and 10 month postfertilization (mpf). As the functional unit of female ovary, follicles develop by a continuous production of preovulatory follicles and follicles of each stage (primordial, transitional, primary, activated primary, antral follicle). Adult ovaries show various stages of oogenesis: stage Ia, Ib, II, III, and IV oocytes. k and m show mature testis at 77 days post fertilization and 1 year post fertilization, respectively. The tubular compartment or testicular cord (Tc) is composed of clusters of germ cells enclosed by a layer of Sertoli cells. K and M show germ cells at various stages of spermatogenesis. Key: Spermatogonia (Sg), spermatocytes (Sc), spermatids (Sd) and sperm (Sp). Scale bar =50 μm (a,b,l) and 100 μm (c,d,e,f,g,h,I,m,n). |
|
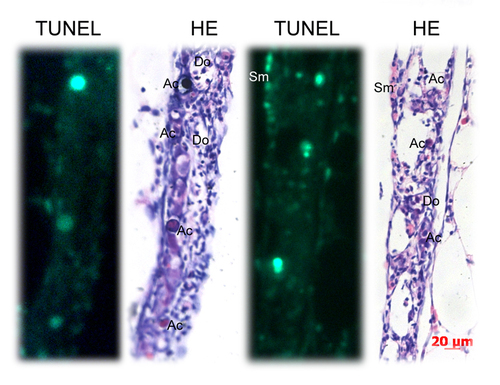
Identification of apoptotic cells by TUNEL staining assay and HE staining. TUNEL-positive cells partially matched with the apoptotic-like cells (Ac) identified by HE staining. Many apoptotic-like cells (Ac) were TUNEL-negative. Many somatic cells (Sm) were TUNEL-positive. Key: Scale bars =20 μm |
|
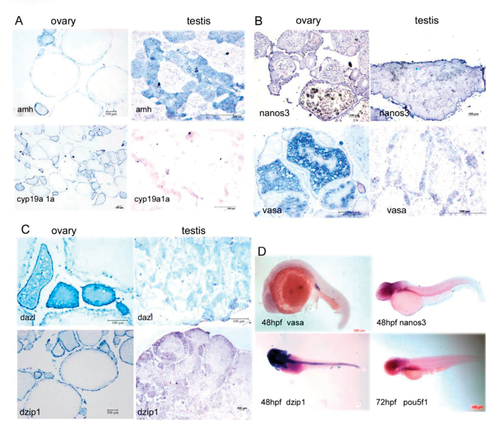
Comparison of the sex determination-associated genes expression patterns in adult gonads (A-C) and in embryos (D) using histo-in situ hybridization. A, amh vs. cyp19a1a; B, nanos3 vs. vasa; C, dzip1 vs. dazl; D, Expression pattern of vasa, nanos3, dzip1 and pou5f1 during embryonic development using whole mount in situ hybridization. |
|
Histo-immunogical analyses of juvenile transforming gonads. Juvenile gonads (35 dpf) were sliced and co-stained with Mek1/2 and pMek1/2 antibodies or co stained with sox9a and p53 antibodies. Blue color is stained by DAPI. HE indicates HE staining. Scale bars= 20 μm. |
|
cAMP-MAPK pathways involved in cellular morphology. Sliced ovary tissues were cultured in in the presence or absence of MKI (PD98059 and U0216 mix) with or without cAMP. |