- Title
-
beta-catenin/Wnt signaling controls progenitor fate in the developing and regenerating zebrafish retina
- Authors
- Meyers, J.R., Hu, L., Moses, A., Kaboli, K., Papandrea, A., and Raymond, P.A.
- Source
- Full text @ Neural Dev.
|
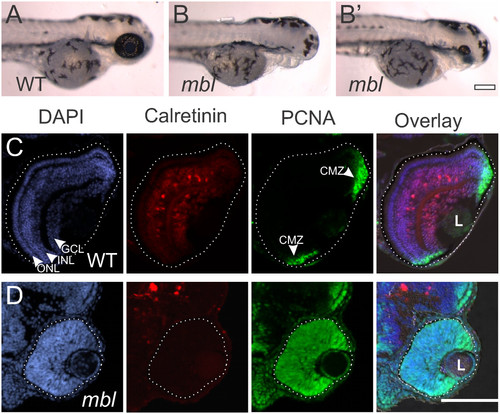
Small retinas in masterblind fish fail to differentiate. Compared with wild-type (WT) fish at 72 hours post fertilization (hpf) (A), masterblind (mbl) fish typically lack forebrain and eyes (B), although some fish develop with a small eye (B’). Cryosections of wild-type retinas at 72 hpf (C) reveal lamination of the nuclei, labeled with DAPI in blue, into three layers: the ganglion cell layer (GCL) nearest the lens (L), the inner nuclear layer (INL), and the outer nuclear layer (ONL). Calretinin-labeled neurons have differentiated in the GCL and INL. Proliferative cells, marked with proliferating cell nuclear antigen (PCNA) are found only in the periphery of the retina in the ciliary marginal zone (CMZ). In contrast, cryosections of mbl retinas at 72 hpf (D) show a lack of nuclear lamination, a failure of calretinin-positive neurons to differentiate, and all cells within the retina remain PCNA-positive. In these and all following figures, the eye is outlined with a dotted line to distinguish it from adjacent tissues. Scale bar: A, B, 200 µm; C, D, 100 µm. |
|
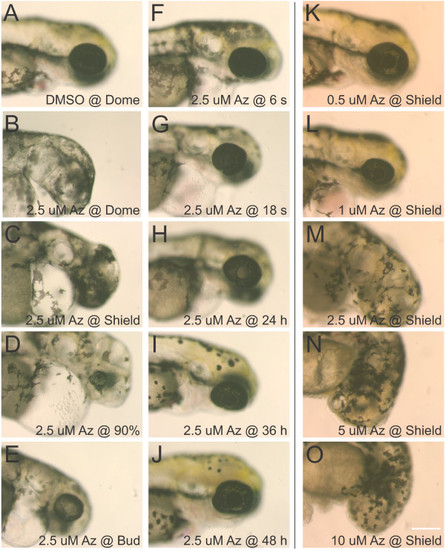
Dose and timing-response for 1-azakenpaullone on retinal development. The GSK3β inhibitor 1-azakenpaullone was applied to embryos at various stages of development and observations were made at 72 hpf. While control embryos treated with 0.1% DMSO as early as dome stage developed normally (A), treatment with 2.5 µM 1-azakenpaullone (Az) at dome or shield stage resulted in loss of forebrain and eyes (B, C). Treatment with 2.5 µM 1-azakenpaullone at 90% epiboly resulted in a small eye lacking a lens (D). At bud-stage or later, treatment with 1-azakenpaullone led to a small eye, with the size of the eye increasing with progressively later treatments (E-J). The effects of 1-azakenpaullone are dose-dependent: embryos treated at shield stage with 0.5 µM 1-azakenpaullone have relatively normal development (K), 1 µM results in a small eye (L), and treatments at 2.5 µM or higher results in failure of eye development (M-O). Scale bar = 200 µm. |
|
Inhibition of GSK3β blocks retinal differentiation and maintains proliferative progenitors.Tg(gfap:GFP)mi2002 zebrafish embryos were treated with 0.025% DMSO as a vehicle control or 2.5 µM 1-azakenpaullone to inhibit GSK3β at various times during development. Retinal cryosections from DMSO-treated embryos show normal development of Müller glia (MG; GFP under control of a glial-specific promoter), photoreceptors (the zpr-1 antibody marks red-green double cones (DC)), and calretinin-positive neurons in the ganglion cell layer (GCL) and INL (A, D). The fluorescent signal in the lens (L) is non-specific. Proliferative cells marked with PCNA are found at the retinal margins in the CMZ (D). In contrast, retinas from embryos treated with 1-azakenpaullone from 12 hpf show no expression of GFAP, Zpr-1 labeled photoreceptors, or calretinin (B, E). Nuclear lamination is absent in these retinas, and proliferative cells labeled with PCNA extend throughout the retina (E). Treatment from 36 hpf allows more differentiation to occur, and the central retina is laminated (C). However, the CMZ, which contains proliferative cells, is expanded (F). Scale bar = 100 µm. |
|
Inhibition of GSK3β leads to expansion of retinal progenitor markers. (A, B)In situ hybridization with an rx1 riboprobe. Control zebrafish embryos at 72 hpf express rx1 in the CMZ at the retinal margin (A; bracket). In contrast, embryos treated with 1-azakenpaullone from 24 hpf to 72 hpf show a marked expansion in the domain of expression of rx1, marking an expanded CMZ (B; bracket). (C, D) Sections through retinas labeled via in situ hybridization with a sox2 riboprobe. Control retinas show sox2 expression within the CMZ (arrows) and in developing amacrine cells (C). 1-azakenpaullone-treated retinas have sox2 expression throughout the retina (D). (E, F) Sections through retinas labeled via in situ hybridization with a vsx2 riboprobe. Control retinas show vsx2 expression within the CMZ (E; arrows). 1-azakenpaullone-treated retinas have vsx2 expression throughout the retina (F). |
|
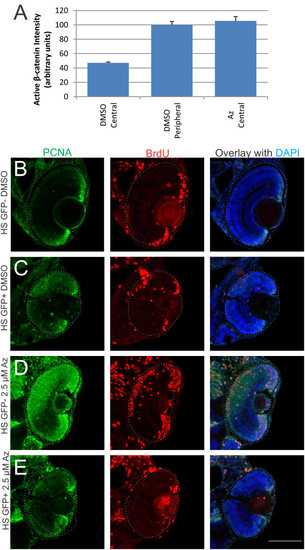
Effects of 1-azakenpaullone are mediated via Wnt/β-catenin pathway. (A) Quantification of fluorescent intensity from an anti-dephosphorylated (active) β-catenin antibody in retinal sections from fish treated with DMSO (vehicle control) or 1-azakenpaullone from 24 to 48 hpf. Average intensity was calculated from approximately 750 µm2 area either in central retina or peripheral retina. DMSO-treated controls show significantly more active β-catenin in peripheral retina compared to central retina, while fish treated with 1-azakenpaullone have elevated levels of active β-catenin in central retina, comparable to that in control-treated peripheral retina. (B-E) Dominant-negative TCF3 suppresses the expansion of the CMZ induced by GSK inhibition. Tg(hsp70:ΔTCF3-GFP)w26 zebrafish embryos, which express a dominant-negative TCF3 (dnTCF) under control of the heat shock promoter, and their wild-type siblings were heat shocked at 36 hpf for 1 h at 39.5°C and then treated with either 0.025% DMSO or 2.5 µM 1-azakenpaullone. Fish were given a pulse of BrdU 2 h prior to fixation at 3 dpf. (B) Wild-type siblings treated with DMSO show a normal CMZ, with BrdU-positive and PCNA-positive cells at the retinal margin. (C) DMSO-treated fish expressing dnTCF showed a decrease in the number of cells at the retinal margin that were BrdU/PCNA immunoreactive. (D) In wild-type sibling fish treated with 1-azakenpaullone, there is a dramatic expansion of the proliferative progenitors marked with PCNA and BrdU. (E) Transgenic fish expressing dnTCF and treated with 2.5 µM 1-azakenpaullone show no expansion of the CMZ, with few dividing cells at the retinal margin, indicating that dnTCF suppresses the 1-azakenpaullone induced expansion of the progenitor pool. Scale bar = 100 µm. |
|
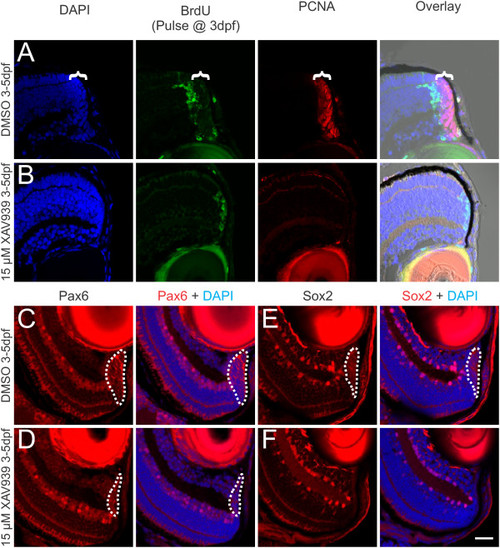
Blocking Wnt signaling with XAV939 leads to loss of the progenitor population in the CMZ. Zebrafish at 3 dpf were given a pulse of BrdU to mark dividing cells, then exposed to either 0.15% DMSO as a vehicle control or 15 µM XAV939 for 2 days and fixed for analysis at 5 dpf. In control fish at 5 dpf, the progeny of cells labeled with BrdU at 3 dpf had differentiated and were displaced out of the CMZ and a population of dividing cells that express PCNA remain in the CMZ (bracket) at 5 dpf (A). In fish treated with 15 µM XAV939, the progeny of the cells labeled with BrdU at 3 dpf remain at the retinal margin and there are no PCNA-labeled cells (B). Overlays in A and B are shown including a brightfield image to allow visualization of the RPE marking the edge of the retina. Labeling control (C, E) or XAV939-treated (D, F) fish with antibodies against Pax6 (C, D) or Sox2 (E, F), shows that XAV939 greatly reduces the number of cells expressing these progenitor cell markers (dotted line marks the extent of labeling in the CMZ). Scale bar = 20 µm. |
|
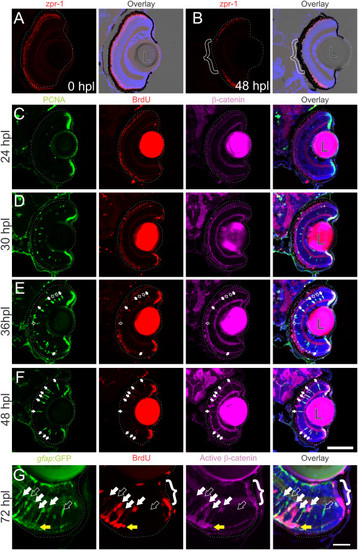
Wnt signaling is activated as Müller glia reenter the cell cycle following intense-light-induced destruction of photoreceptors.Tg(gfap:GFP)mi2002 zebrafish larvae were exposed to intense light at 6 dpf to lesion their photoreceptors. Immediately following exposure, the retina appeared normal, with photoreceptors (double cones labeled with zpr-1) throughout the ONL (A). Within 48 hours post lesion (hpl), photoreceptors are missing from central retina as shown by the lack of zpr-1 staining (B; bracket). (C-F) Light-lesioned fish were incubated continuously in 2.5 mM BrdU and fixed at 24, 30, 36, or 48 hpl. Cells in the INL become PCNA-positive between 24 and 30 hpl, and begin to incorporate BrdU around 36 hpl (C-E). Immunoreactivity for β-catenin is absent from the INL at 24 or 30 hpl (C, D), but begins to accumulate by 36 hpl (E), when all BrdU-positive, PCNA-positive cells (white arrows) and some BrdU-negative, PCNA-positive cells (open arrows) are immunoreactive. By 48 hpl, all of the PCNA-positive cells are also BrdU-positive and β-catenin-positive (F; white arrows). At 72 hpl, BrdU-positive cells in the INL also express GFP, indicating their Müller glial origins, and they are co-labeled with an antibody for dephosphorylated (active) β-catenin (white arrows; G). Proliferative cells are strongly immunoreactive for dephosphorylated β-catenin (open arrow). The cells of the CMZ also exhibit strong immunoreactivity for dephosphorylated β-catenin (bracket). Scale bars A-F: 100 µm; G: 50 µm. |
|
Inhibition of Wnt signaling with dnTCF or XAV939 inhibits injury-induced proliferation. (A, B) dnTCF zebrafish larvae and wild-type siblings were given an intense light lesion at 6 dpf and heat shocked at 39.5°C for 1 h at 1 dpl and again at 2 dpl then fixed at 3 dpl. In wild-type siblings, PCNA-labeled proliferative cells are in the CMZ (arrows), INL, and ONL at 3 dpl (A), while dnTCF zebrafish have few proliferative cells in either the retina or the CMZ (B). (C, D) Wild-type zebrafish lesioned as in (A, B) were treated with 0.15% DMSO or 15 µM XAV939, with 2.5 mM BrdU present continuously in the media until fixation at 3 dpl. In DMSO-treated fish, BrdU-positive cells are in the INL and ONL (C), while XAV939-treated fish have few proliferative cells in either the INL or ONL (D). Scale bar = 50 µm. |
|
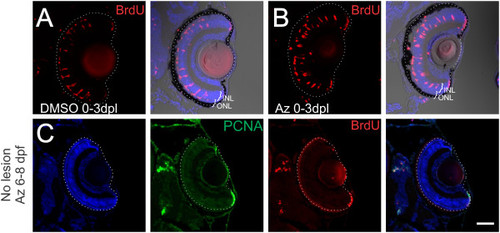
Inhibition of GSK3β does not alter number of dedifferentiating Müller glia. Zebrafish larvae were exposed to intense-light at 6 dpf and incubated in BrdU with either DMSO as a vehicle control or 1-azakenpaullone from 0 to 3 dpl. (A, B) Light-lesioned fish treated with DMSO or 1-azakenpaullone from 0 to 3 dpl had no difference in the number of BrdU-positive cells in the INL and in the ONL. (C) Zebrafish that did not have a light lesion were soaked in 2.5 µM 1-azakenpaullone from 6 to 8 dpf to test whether it was sufficient to induce Müller glial proliferation in the absence of a lesion. We saw no PCNA or BrdU-positive nuclei in unlesioned fish. Scale Bar = 100 µm. |
|
Inhibition of GSK3β results in delayed loss of proliferative Müller glia.Tg(gfap:GFP)mi2002 zebrafish larvae were exposed to intense-light at 6 dpf and incubated in BrdU with either DMSO as a vehicle control or 1-azakenpaullone from 1 to 5 dpl or 3 to 5 dpl. (A) Light-lesioned fish treated with DMSO from 1 to 5 dpl have BrdU-positive cells in the INL and in the ONL, and a normal distribution of GFP-labeled Müller glia in the INL. (B) In light-lesioned fish treated with 1-azakenpaullone from 1 to 5 dpl, BrdU-positive cells are only in the ONL, with few-to-none in the INL. Additionally, fewer GFP-labeled Müller glia are present compared to controls (see results). (C) Light-lesioned zebrafish treated with BrdU from 1 to 5 dpl and 1-azakenpaullone from 3 to 5 dpl also have few or no BrdU-positive cells in the INL and the number of Müller glia is reduced. (D, E) High magnification views of BrdU-positive GFP-positive cells that accumulated in the ONL at 3 dpl. (D) In fish treated with DMSO from 0 to 3 dpl, clusters of GFP-positive, BrdU-positive Müller glia and their progeny are distributed between both the ONL and the INL (dotted line indicates the outer plexiform layer separating the ONL and the INL). Arrows indicate the GFP-labeled basal processes of radial Müller glia. (E) In fish treated with 2.5 µM 1-azakenpaullone from 0 to 3 dpl, most of the BrdU-positive GFP-positive cells are in the ONL, and the radial processes of Müller glia are absent. Scale bar: A-C, 100 µm; D, E, 20 µm. |
|
Further evidence that inhibition of GSK3β blocks retinal differentiation and maintains proliferative progenitors. A pulse of BrdU provided 2 h prior to fixation at 72 hpf of fish treated with DMSO starting at 12 hpf shows the progenitors at the CMZ are actively moving through S-phase, while at the back of the retina zpr3 labels the rod photoreceptors (A). Treatment with 2.5 µM 1-azakenpaullone beginning at 12 hpf prevents rod differentiation and BrdU-positive cells are found throughout the retina (B). Treatment with 1-azakenpaullone beginning at 24 or 36 hpf allows some rods to differentiate, though there are still expanded pools of proliferating progenitors (C, D). Fish treated with 1-azakenpaullone beginning at 24 hpf also have expanded PCNA-positive domains, loss of calretinin-positive neurons, loss of GFP-positive Müller glia, and loss of zpr-1 labeled double cones (E, F). Inhibition of GSK3β with a 1 h treatment of 0.3 M LiCl at 48 hpf shows effects on retinal development similar to treatment with 1-azakenpaullone, with a reduced number of calretinin-positive neurons and an expanded CMZ (PCNA-labeled) compared with controls treated with 0.3 M NaCl (G,H). Scale bar. |