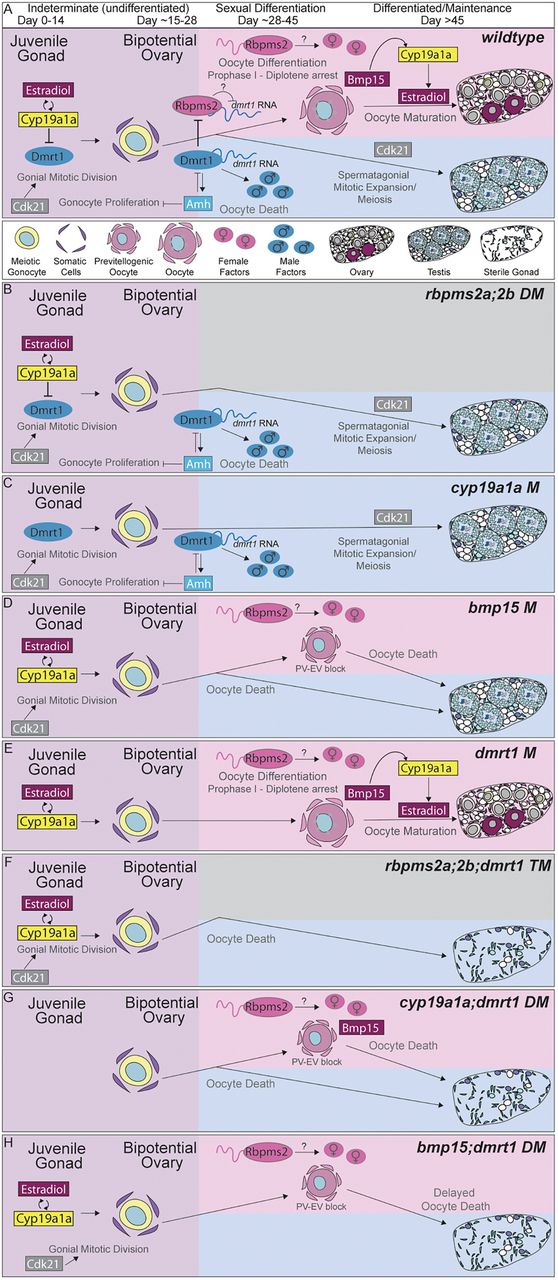
Fig. 6 Model: Cyp19a1a inhibits dmrt1 to promote bipotential ovary formation and acquisition of female fates, which requires rbpms2 even in the absence of dmrt1. (A-F) Timeline (from left to right) of requirement for each essential regulator of sex determination. (A) Cyp19a1a is required for the transition from juvenile gonad to bipotential ovary by antagonizing Dmrt1 activity. Cdk21 promotes mitotic divisions of gonocytes, whereas Amh limits gonial proliferation within the bipotential ovary. Inhibition of Dmrt1 allows expression of Rbpms2, either directly or indirectly, and female development in the absence of Cyp19a1a. Rbpms2 probably promotes female fates temporally upstream of Bmp15 and estrogen (estradiol), which promote follicle maturation beyond pre-vitellogenic phases. Cyp19a1a is an enzyme that functions to catalyze the final step of estrogen synthesis; therefore, the antagonistic relationship between Cyp19a1a and Dmrt1 is probably an indirect relationship mediated by the estrogens produced by Cyp19a1a. Although Cyp19a1a is dispensable to establishment of the bipotential ovary and initiation of female development in the absence of Dmrt1, it appears to be required for subsequent oocyte maturation and maintenance of female fates independently of antagonism of Dmrt1. Bmp15 produced in oocytes is required to promote oocyte differentiation and cyp19a1a-positive granulosa cells, indicating that the subsequent failure of cyp19a1a;dmrt1 double mutant oocytes to mature is a result of loss of the later granulosa cell-derived source of Cyp19a1a, which appears not to require inhibition of Dmrt1. (B-H) Model of mutant scenarios for each essential regulator. (B) In rbpms2a;rbpms2b double mutants, Rbpms2 repression of dmrt1 transcript is alleviated and Dmrt1 is translated, leading to male differentiation. Within the testis, Cdk21 promotes spermatogonial expansion and meiosis. (C) In cyp19a1a mutants, Cyp19a1a no longer antagonizes dmrt1, probably due to reduced estradiol synthesis, and Dmrt1 is expressed prematurely, leading to precocious male differentiation. (D) In bmp15 mutants, oocytes do not progress past the PV stage, in part because Bmp15 is required for late Cyp19a1a production. (E) In dmrt1 mutants, Dmrt1 is not present to antagonize Rbpms2, and female differentiation ensues. (F) In rbpms2a;rbpms2b;dmrt1 triple mutants, female differentiation cannot occur in the absence of Rbpms2, whereas male differentiation cannot proceed because of loss of Dmrt1, resulting in the formation of a sterile testis. (G) In cyp19a1a;dmrt1 double mutants, loss of dmrt1 is sufficient to restore the transition from juvenile gonad to bipotential ovary and to prevent precocious male differentiation as occurs in cyp19a1a mutants. However, despite the absence of dmrt1, oocytes lacking all Cyp19a1a (cyp19a1a;dmrt1 double mutants) or (H) Bmp15-dependent Cyp19a1a (bmp15;dmrt1 double mutants) fail to progress to vitellogenic stages and female development ultimately fails, indicating that Cyp19a1a promotes oocyte progression independently of dmrt1 antagonism. Probably because of later requirements for dmrt1 in testis development, loss of both dmrt1 and cyp19a1a or bmp15 eventually results in sterile male adults.
Image
Figure Caption
Acknowledgments
This image is the copyrighted work of the attributed author or publisher, and
ZFIN has permission only to display this image to its users.
Additional permissions should be obtained from the applicable author or publisher of the image.
Full text @ Development