Fig. S1
Generation of TEAD reporter, yap1 mutant, and wwtr1 mutant zebrafish, Related to Figure 1.
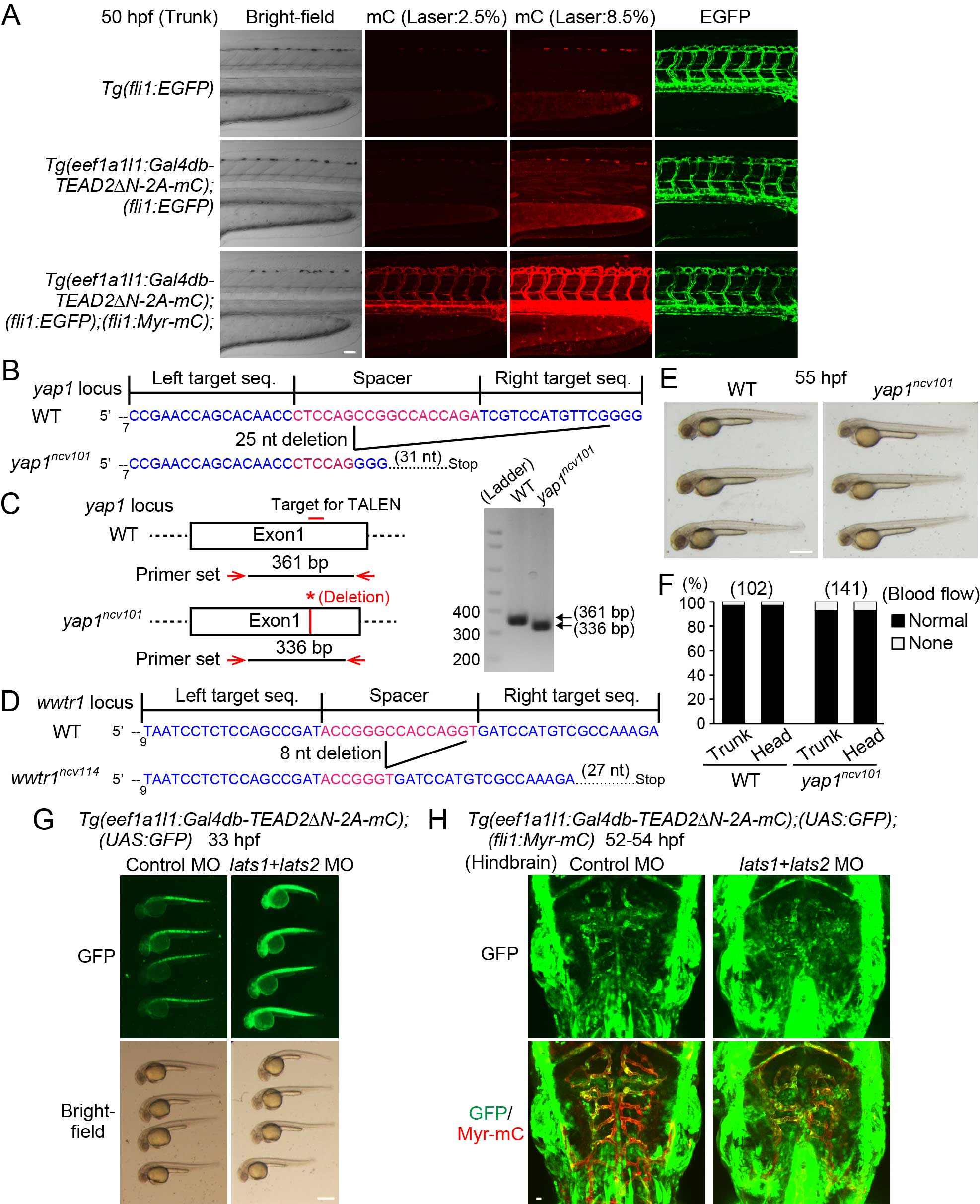
(A) Projection view of confocal stack fluorescence images of Tg(fli1:EGFP) (top), Tg(eef1a1l1:Gal4db-TEAD2ΔN-2A-mC);(fli1:EGFP) (middle), and Tg(eef1a1l1:Gal4db-TEAD2ΔN-2A-mC);(fli1:EGFP);(fli1:Myr-mC) (bottom) embryos at 50 hpf. Lateral views, anterior to the left, unless otherwise described. Left column, bright-field images; the second and third columns, mC images taken with different laser transmissivity of 559 nm laser indicated at the top; right column, EGFP images. While fluorescence of mC of Tg(eef1a1l1:Gal4db-TEAD2ΔN-2A-mC) was ubiquitously detected at stronger laser illumination (8.5%), it was negligibly faint compared to that of Myr-mC of Tg(fli1:Myr-mC) at weaker laser illumination (2.5%).
(B) Schematic diagrams of the wild-type yap1 (WT) (upper) and mutant yap1ncv101 (lower) alleles. TALENs were designed to target exon1 of yap1. The left and right TALEN binding sites are shown in blue. The spacer region is shown in red. The yap1ncv101 allele harbors a 25 nucleotide (nt) deletion in the first exon, resulting in a premature stop codon.
(C) Genotyping of single embryo sampled from WT and homozygous yap1ncv101 mutant fish. PCR analyses of genomic DNAs were performed using a primer set indicated in the left panel. The 361-bp fragment and the 336-bp fragment were PCR-amplified in the WT and yap1ncv101 embryo, respectively.
(D) Schematic diagrams of the WT allele (upper) and mutant wwtr1ncv114 allele (lower) are shown similarly to (B). The wwtr1ncv114 allele harbors an 8 nucleotide (nt) deletion in the first exon, resulting in a premature stop codon.
(E) Bright-field images of WT and homozygous yap1ncv101 mutant embryos at 55 hpf. Note that the yap1ncv101 mutant embryos are morphologically normal.
(F) Graph shows percentage of the number of embryos showing normal circulation (Normal) and no circulation (None) in the trunk or head region of WT or homozygous yap1ncv101 mutant embryos (at 55 hpf) among the total numbers of embryos observed (indicated at the top). Note that the yap1ncv101 mutant embryos have normal circulation.
(G) Stereomicroscopic images of Tg(eef1a1l1:Gal4db-TEAD2ΔN-2A-mC);(UAS:GFP) embryos (33 hpf) injected with control morpholino oligonucleotide (MO) (left) or lats1 and lats2 double MOs (right). Note that GFP expression is markedly enhanced in the entire embryo injected with the lats1 and lats2 double MOs. Similar results were obtained in 2 independent experiments.
(H) Projection view of confocal stack fluorescence images of the hindbrain in Tg(eef1a1l1:Gal4db-TEAD2ΔN-2A-mC);(UAS:GFP);(fli1:Myr-mC) embryos (52-54 hpf) injected with control MO (left) or lats1 and lats2 double MOs (right). Dorsal view, anterior to the top. Upper, GFP images (green); lower, the merged images (GFP, green; Myr-mC, red). Note that GFP expression is enhanced both in ECs and non-ECs by the injection of the lats1 and lats2 double MOs. Representative images of 2 independent experiments are shown. Scale bars, 50 µm in (A), 500 µm in (E) and (G), and 10 µm in (H).
Reprinted from Developmental Cell, 40, Nakajima, H., Yamamoto, K., Agarwala, S., Terai, K., Fukui, H., Fukuhara, S., Ando, K., Miyazaki, T., Yokota, Y., Schmelzer, E., Belting, H.G., Affolter, M., Lecaudey, V., Mochizuki, N., Flow-Dependent Endothelial YAP Regulation Contributes to Vessel Maintenance, 523-536.e6, Copyright (2017) with permission from Elsevier. Full text @ Dev. Cell