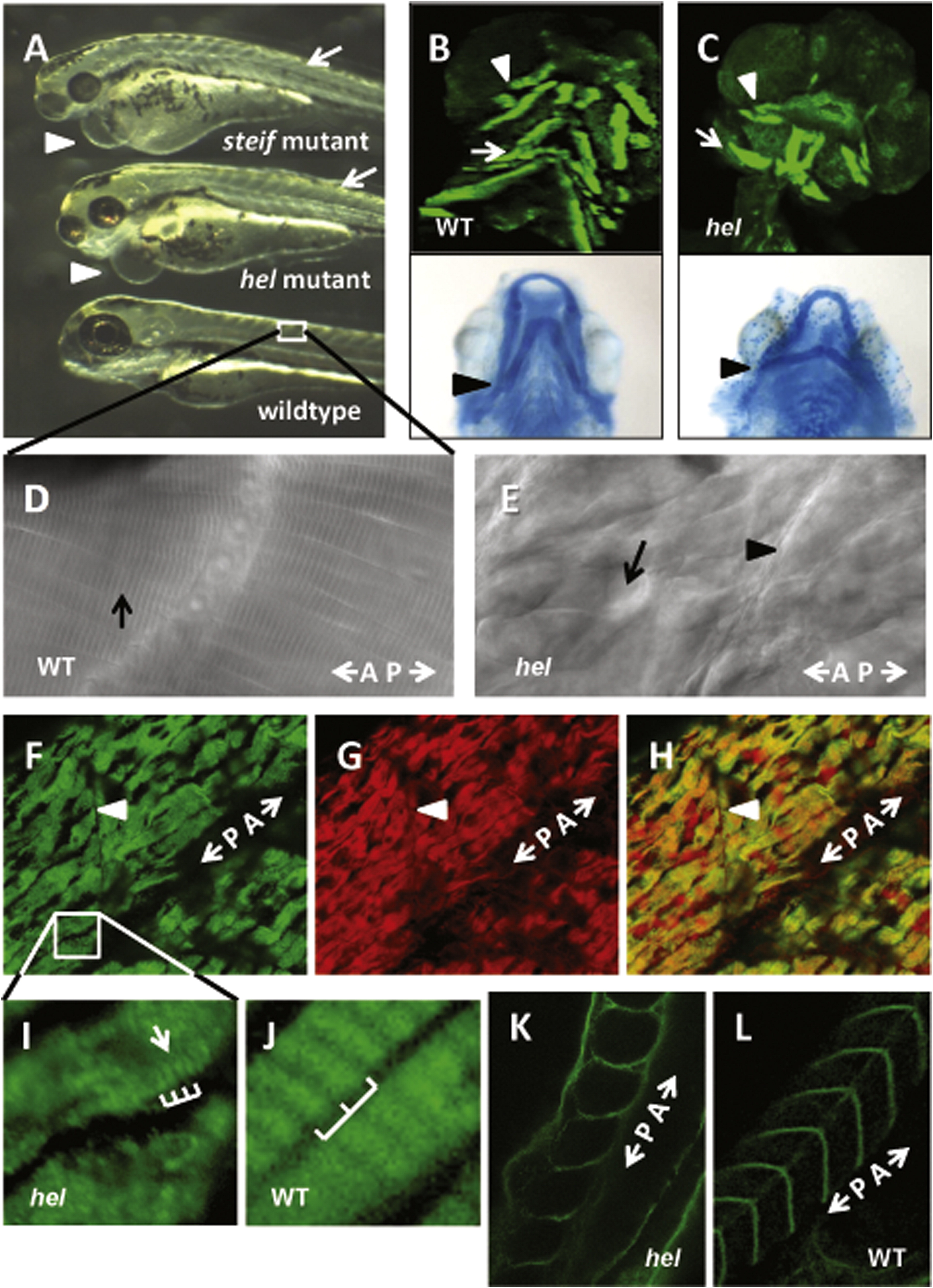
Fig. 3 The mutant phenotype of herzschlag is characterized by circulation and edema-related defects, shortened myofibers and disorganized sarcomeres. Comparison of the hel mutant with other motility mutants (steif) at 3 days post-fertilization (dpf) demonstrates the common phenotypes (A), including heart edema (arrowheads), small head and eyes, and reduced organization of trunk musculature (arrows). Reduced heartbeat in motility mutants results in pericardial edema and dysplasia of muscular (B and C, upper panels) and skeletal (lower panels) elements of the head by 5 dpf. Compared with wild-type embryos (B), hel mutants (C) have smaller occulomotor muscles (arrows), laterally displaced interhyoideus muscles (arrowheads) and ceratohyal cartilages (lower panel, arrowheads), and reduced or absent hyoideus muscles. DIC imaging of somites in 5 dpf hel embryos (F) shows a complete loss of myocyte striations as compared to WT (arrow in D), with shortened myofibers separated by vacuole-like spaces (arrow in E). A–P indicates the direction of the anterior–posterior axis. Immunostaining for thick filament myosin (F) and thin filament actin (G) at 3 dpf demonstrates the extent of myofibril disorganization, with greatly shortened myofibers and lateral separations between cells. (H) A merged image shows co-localization of myosin and actin in mutant embryos. At higher magnification (I and J), it can be seen that some myosin periodicity (arrow) seems to remain in hel mutants (bars in I), but at a much shorter period than in wild-type embryos (bars in J). The integrity of myosepta was not affected by the hel mutation (arrowheads in E and F). This is particularly evident following immunostaining of extracellular laminin in WT (K) and hel mutant embryos (L). In all experiments, a minimum of 20 embryos were examined to ensure consistency of the phenotype.
Reprinted from Developmental Biology, 387(1), Myhre, J.L., Hills, J.A., Prill, K., Wohlgemuth, S.L., and Pilgrim, D.B., The titin A-band rod domain is dispensable for initial thick filament assembly in zebrafish, 93-108, Copyright (2014) with permission from Elsevier. Full text @ Dev. Biol.