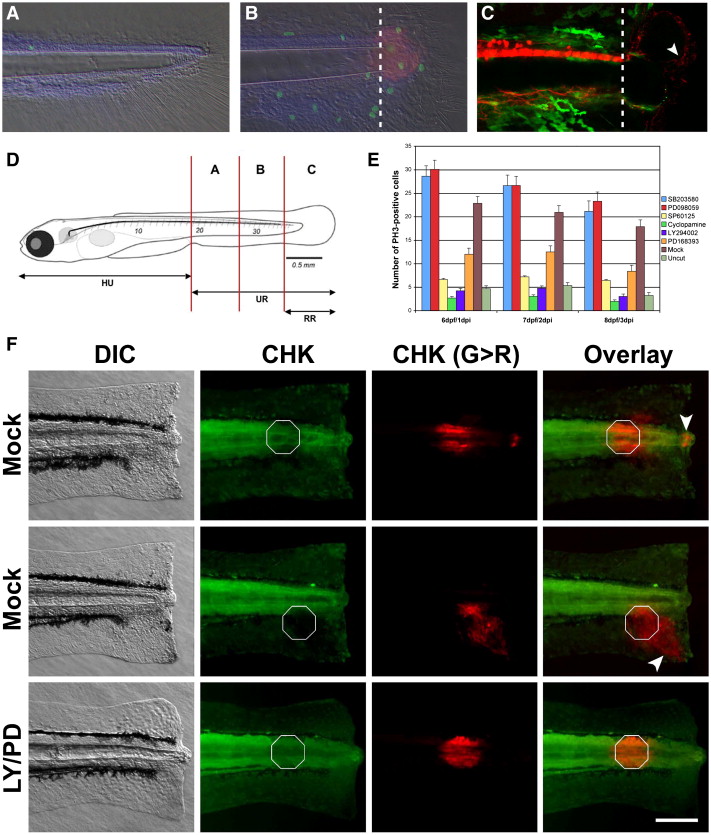
Fig. 1 A chemical genetic screen in zebrafish identifies several oncogenes as regulators of epimorphic regeneration. (A) Zebrafish larvae are able to regenerate a significant portion of the tail that includes spinal cord, muscle, skin and blood vessels. At 6 dpf very few proliferating cells (visualized in green with anti-Phospho Histone H3 antibodies) are detected in the caudal region of control larvae. (B) Conversely, a marked increment of mitotic cells can be observed in amputated larvae at 6 dpf/1 dpi. Some, but not all of the dividing cells are present in the blastema (visualized in red with an antibody anti-Vimentin). (C) Transgenic lines NBT:DsR2 (van Drenth, M., unpublished) and FoxD3:EGFP indicate that the blastema is heavily innervated (white arrowhead, red channel) and void of neural crest cells at 1 dpi (green channel). Dotted lines indicate the amputation plane in panels (B) and (C). Amputation-induced proliferation of progenitor cells can be modulated by small chemical compounds. (D) In order to quantify the proliferative response, the number of dividing cells is counted in each one of the compartments depicted and (E) tabulated in the presence or absence of small compound inhibitors specific for a number of oncogenes. Data for the regions A + B (see panel D) is shown (E). At 6 dpf/1 dpi, the number of proliferating cells in region B is significantly enhanced by incubating regenerating larva in 20 μM SB203580 and 20 μM PD098059. In contrast, this number is significantly reduced in larva incubated in 20 μM SP600125, 5 μM Cyclopamine, 5 μM LY294002 or 10 μM PD168393. (F) Different cell types migrate towards the amputation plane during regeneration. Larvae were amputated at 5 dpf followed by immediate photoconversion of the fluorescent protein CoralHue™ Kaede at localized spots (white octagons). At 24 hpi different cell types, including cells from the epidermis and the notochord (white arrowheads), move towards the amputation plane in larvae incubated in DMSO. Conversely, no cell movement was observed in larvae incubated in 10 μM PD168393/LY294002 during the same period of time. Importantly, cells from uncut larvae do not undergo migration over the time window of our experiments (data not shown). Under our conditions, UV light photoconverts many cells along the L/R axis at the given A/P position in the larvae. This is useful for the main reason that as most photoconverted cells do not move towards the amputation plane they clearly identify the initial A/P position of the photoconverted cluster at 24 hpi (as indicated by the white octagons in the panel). For the purpose of morphological reference: HU, head to urogenital region; UR, urogenital region; RR, regenerated region. Scale bar 100 μm.
Reprinted from Developmental Biology, 327(1), Rojas-Muñoz, A., Rajadhyksha, S., Gilmour, D., van Bebber, F., Antos, C., Rodríguez Esteban, C., Nüsslein-Volhard, C., and Izpisúa Belmonte, J.C., ErbB2 and ErbB3 regulate amputation-induced proliferation and migration during vertebrate regeneration, 177-190, Copyright (2009) with permission from Elsevier. Full text @ Dev. Biol.