- Title
-
Investigating the role of phenylalanine residues for amyloid formation of the neuropeptide neurokinin B
- Authors
- Jayawardena, B.M., Azzi, A., Jones, C.E.
- Source
- Full text @ Biochem. Biophys. Res. Commun.
|
Amyloidogenic properties of NKB. A. Predicted aggregation propensity (using TANGO) of NKB(WT) and the mutants investigated in this study. B. Time-resolved ThT fluorescence of NKB(WT) and mutants NKB(F6W), NKB(F5W) and NKB(F5,6W) shows that mutations in the diphenylalanine motif severely disrupt the ability of the peptide to form fibrils. C. Time-resolved ThT fluorescence of NKB(+A6) which inserts an alanine within the diphenylalanine motif shows limited fibril formation. D. Time-resolved ThT fluorescence of the zebrafish NKB (zf-NKB) shows some fibril formation, but much less compared to mammalian wild-type NKB. All peptides were 200 μM in 10 mM nEM, pH 7.4. |
|
Electron microscopic analysis of fibrils formed by A. NKB(WT); B. NKB(F6W); C. NKB(+A6) and D. zf-NKB. On the right of each microscope image is the width distribution of the fibrils estimated from EM images. The mean ± SEM of this data is presented in the main text. Scale bar = 100 nm. |
|
Formation of an intermolecular disulfide bond restores amyloid formation. Time-resolved ThT fluorescence shows NKB(F6C) forms few fibrils when in reducing conditions (NKB(F6C), R), but when in non-reducing conditions, (NKB(F6C), NR), ThT-positive fibrils are generated. Fibril formation by wild-type NKB (NKB(WT) is shown for comparison. Peptides were 200 μM in 10 mM nEM, pH 7.4. |
|
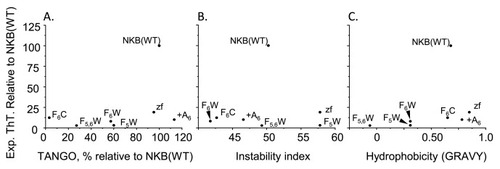
Pairwise comparison between TANGO predicted aggregation propensity and experimental fibril formation and the physicochemical parameters of B. instability index and C. GRAVY hydrophobicity index with the experimental fibril formation for each of the peptides studied. Experimental fibril formation is based on extent of formation of ThT-positive fibrils. |