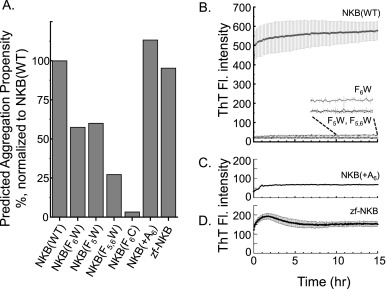
Fig. 1 Amyloidogenic properties of NKB. A. Predicted aggregation propensity (using TANGO) of NKB(WT) and the mutants investigated in this study. B. Time-resolved ThT fluorescence of NKB(WT) and mutants NKB(F6W), NKB(F5W) and NKB(F5,6W) shows that mutations in the diphenylalanine motif severely disrupt the ability of the peptide to form fibrils. C. Time-resolved ThT fluorescence of NKB(+A6) which inserts an alanine within the diphenylalanine motif shows limited fibril formation. D. Time-resolved ThT fluorescence of the zebrafish NKB (zf-NKB) shows some fibril formation, but much less compared to mammalian wild-type NKB. All peptides were 200 μM in 10 mM nEM, pH 7.4.
Image
Figure Caption
Acknowledgments
This image is the copyrighted work of the attributed author or publisher, and
ZFIN has permission only to display this image to its users.
Additional permissions should be obtained from the applicable author or publisher of the image.
Full text @ Biochem. Biophys. Res. Commun.