- Title
-
ANGPTL4 accelerates ovarian serous cystadenocarcinoma carcinogenesis and angiogenesis in the tumor microenvironment by activating the JAK2/STAT3 pathway and interacting with ESM1
- Authors
- Li, Y.K., Gao, A.B., Zeng, T., Liu, D., Zhang, Q.F., Ran, X.M., Tang, Z.Z., Li, Y., Liu, J., Zhang, T., Shi, G.Q., Zhou, W.C., Zou, W.D., Peng, J., Zhang, J., Li, H., Zou, J.
- Source
- Full text @ J Transl Med
|
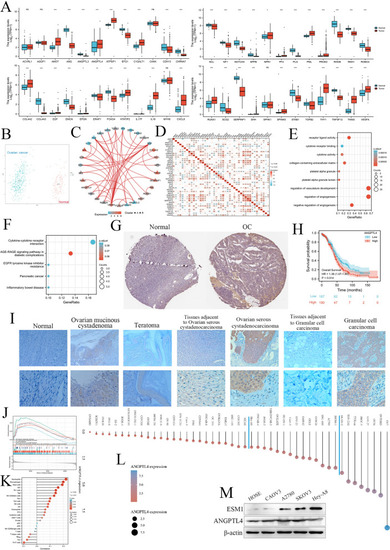
The expression, potential functions, diagnostic value and prognostic value of ARGs in OC patients. |
|
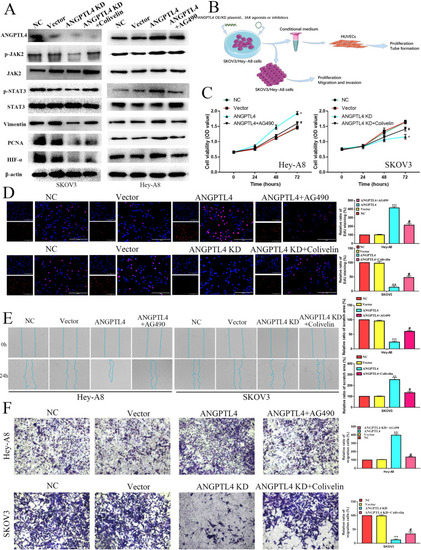
The effects of the ANGPTL4/JAK2/STAT3 pathway on OC proliferation, migration and invasion. |
|
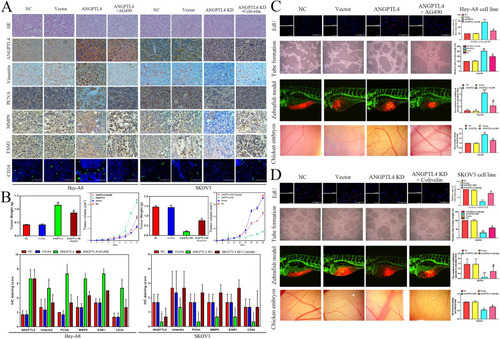
ANGPTL4 promotes OC growth and angiogenesis by activating the JAK2/STAT3 pathway in vivo. |
|
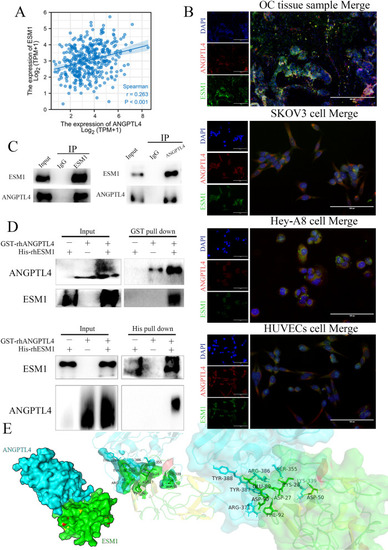
ANGPTL4 interacted with ESM1. |
|
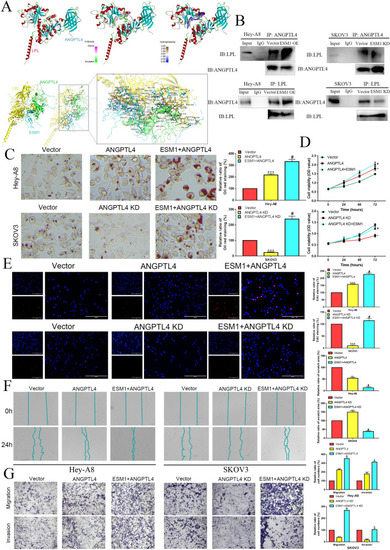
ESM1 promotes ANGPTL4 to combine with LPL by accelerating proliferation, invasion and lipid accumulation in OC cells. |
|
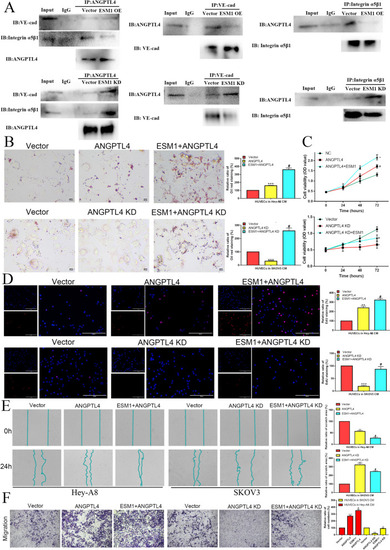
ESM1 inhibits ANGPTL4 binding to integrin α5β1 and VE-cadherin, which represses HUVEC proliferation and migration to induce vascular permeability in the tumor microenvironment. |
|
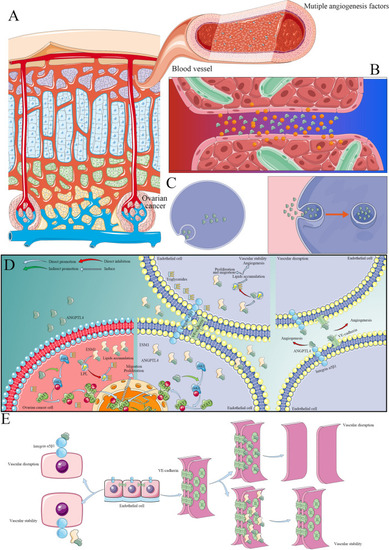
A model of the molecular mechanism by which ANGPTL4 promotes OC development and progression. |