- Title
-
Dynamic and broad expression of adamts9 in developing and adult zebrafish
- Authors
- He, Y., Carver, J.J., Erickson, T., Le Pabic, P., Zhu, Y.
- Source
- Full text @ Dev. Dyn.
|
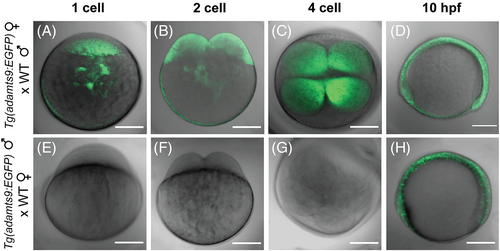
Maternal expression of adamts9 in zebrafish embryos. A–D, Maternally-deposited EGFP signal in progeny from crosses of female Tg(adamts9:EGFP)ecu19 transgenics with wild-type males (WT, AB strain). Merged confocal images (EGFP and T-PMT channels) of embryos at the 1-cell, 2-cell, 4-cell, and 10 hours post-fertilization (hpf) stages are shown. Similar expression was confirmed in multiple F3 embryos from five independent F2 transgenic lines. E,G, No observable EGFP signal prior to 10 hpf in early dividing embryos from crosses of male Tg(adamts9:EGFP)ecu19 transgenics with WT females. H, zygote adamts9:EGFP expression. Scale bar: 200 μm. |
|
adamts9:EGFP expression in various tissues during zebrafish development. A, weak adamts9:EGFP expression was observed in the developing retina and somites at 24 hpf. B, adamts9:EGFP expression at 36 hpf in middle brain. C–I, adamts9:EGFP |
|
Transient adamts9:EGFP expression in the retina during zebrafish development. Representative confocal images of EGFP expression in the retina of Tg(adamts9:EGFP) larvae at 36 or 60 hpf, with or without phenylthiourea (PTU) treatment to suppress melanin pigment formation. EGFP, transmitted light (T-PMT), red fluorescent protein (RFP, for monitoring autofluorescence) or merged channels are shown. Identical confocal settings were used for both 36 and 60 hpf. Similar transient expression was confirmed in multiple F2 embryos from five independent transgenic lines. Scale bar: 50 μm. |
|
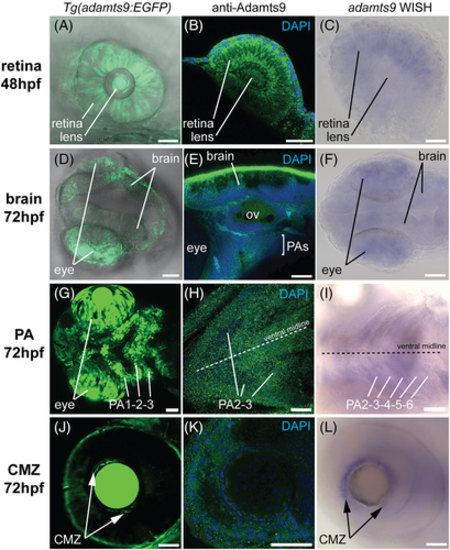
Comparison of adamts9 signal profile detected by EGFP reporter, immunohistochemistry, and whole mount in situ hybridization (WISH) in retina, brain, pharyngeal arches (PAs), and ciliary marginal zone (CMZ) in zebrafish embryos. A–C, expression in retina. D–F, expression in brain. G–I, expression in PAs. J–L, expression in CMZ. Adamts9 protein was detected using Alexa Fluor 488 conjugated secondary antibody (in green color). Nucleus was co-stained with DAPI. Purple color shows positive WISH signal. CMZ, ciliary marginal zone; OV, otic vesicle; PA, pharyngeal arch. Scale bar: 50 μm. |
|
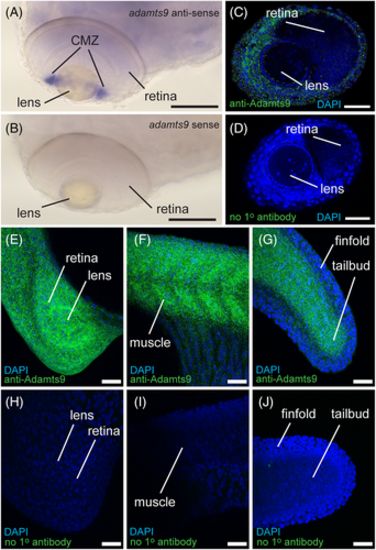
Positive and negative control staining of whole mount in situ hybridization (WISH) and immunohistochemistry (IHC). A,B, WISH signal in ciliary marginal zone (CMZ) of zebrafish embryos at 72 hours post fertilization (hpf) with adamts9- |
|
Strong immuno-signal in wildtype zebrafish embryos. Embryos with mixed genotype (i.e., +/+, +/−, −/−) from adamts9+/− in-cross between 24 and 72 hours post fertilization (hpf) were immune-stained together in the same reaction tubes with an anti-Adamts9 antibody and EGFP labeled second antibody. Each embryo was confocal imaged for EGFP signal and scored for maximum EGFP signal by a blinder observer, then genotyped. Scale bar: 200 μm. |
|
adamts9:EGFP expression in adult tissues of zebrafish. A–H, representative merged confocal images (EGFP and T-PMT channels) of heart (A), pectoral fin (B, scale bar: 200 μm), testis (C, scale bar: 200 μm; D, scale bar: 50 μm), gill (E, scale bar: 200 μm; F, scale bar: 10 μm), and kidney (G,H, scale bar: 50 μm) from a 4-month old mature male zebrafish. I, a representative gel image of RT-PCR analyses (30 cycles) of adamts9 in different tissues of adult zebrafish. J, real-time quantitative PCR (qPCR) analyses of adamts9 expression in different tissues. Results were presented as mean ± SEM (n = 6). Different letters above the error bars indicate that those groups are significantly different at P < 0.05. The expression of adamts9 in preovulatory follicles (stage IVb folic.) was omitted due to its large difference. |
|
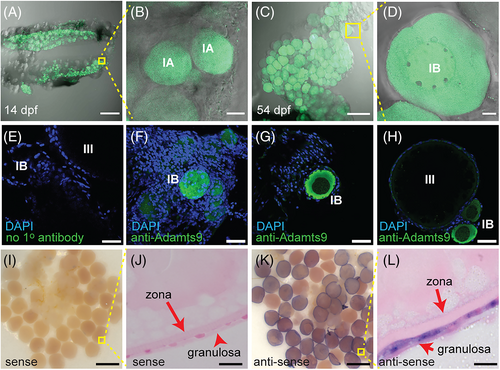
adamts9 signal in adult gonad of zebrafish. A–C, adamts9:EGFP expression. Representative merged confocal images (EGFP and T-PMT channels) of ovaries from adult female zebrafish. No observable EGFP signal in fully-grown but immature follicles (stage IVa, A). Strong EGFP signals were found in preovulatory follicles (stage IVb, B, and C). dpf, days post fertilization. D–I, immunohistochemistry staining with an anti-Adamts9 primary antibody in adult ovaries (D–F) or adult testes (H,I). G, Negative control staining without the primary antibody. Scale bar: 200 μm (A,B); 50 μm (E,G–I); 10 μm (C,D,F). |
|
adamts9 signal in zebrafish ovaries. A–D, adamts9:EGFP expression. Representative merged confocal images (EGFP and T-PMT channels) of ovaries from juvenile female zebrafish. EGFP signal was enhanced due to low level of expression. E, Control immunohistochemistry staining without the primary antibody. F–H, immunohistochemistry staining with an anti-Adamts9 primary antibody. I–L, whole-mount in situ hybridization (WISH) in oocytes. dpf, days post fertilization. Scale bar: 200 μm (A,C); 100 μm (I,K); 50 μm (E–H); 10 μm (J,L). |