- Title
-
Kuoxin Decoction promotes lymphangiogenesis in zebrafish and in vitro based on network analysis
- Authors
- Peng, L., Ma, M., Dong, Y., Wu, Q., An, S., Cao, M., Wang, Y., Zhou, C., Zhou, M., Wang, X., Liang, Q., Wang, Y.
- Source
- Full text @ Front Pharmacol
|
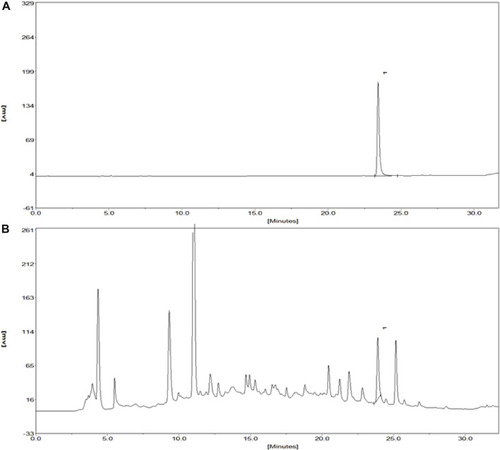
Chromatograms of the Salvianolic acid B standard and extract of KXF. |
|
|
|
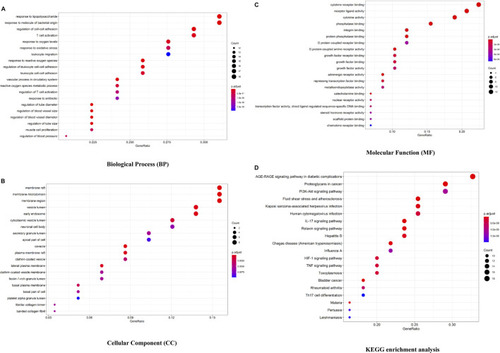
Bioconductor database-enriched pathways and GO entries. |
|
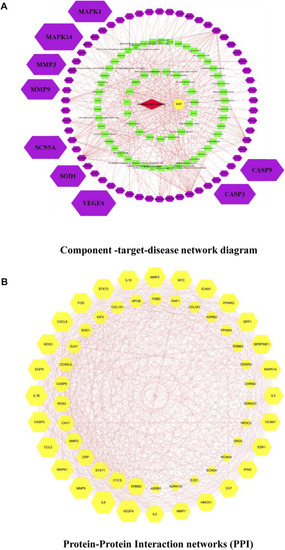
Medicinal material-component-target-signal pathway network. Blue nodes represent the 5 principal components of KXF, purple nodes represent active compounds, red nodes represent relative genes, and yellow nodes represent signaling pathways. 黄芪: |
|
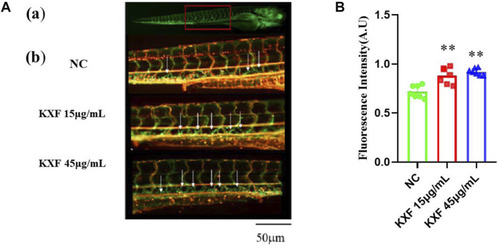
Toxicity analysis of KXF in zebrafish. Tg (Flila: egfp; Gata1: dsred) transgenic zebrafish of 48hpf was treated with different dosages of KXF for 48 h to determine the safe dosage of KXF. Quantitation of the survival zebrafish number. ** |
|
KXF increased the lymphatic thoracic duct formation in zebrafish. |
|
Impaired lymphatic thoracic duct formation induced by VEGFR-3 kinase inhibitor (MAZ51) was rescued by KXF. |
|
KXF promoted the expression of mRNAs related to the lymphatic vessel. The image represents the expression levels of |
|
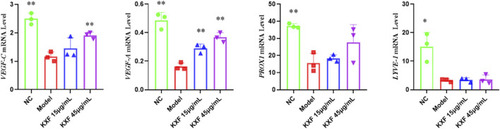
KXF increased the expression of mRNAs associated with lymphatic vessels after zebrafish thoracic lymphatic vessels were damaged by the VEGFR-3 kinase inhibitor (MAZ51). After the pre-treatment of MAZ51 (6 h), the expression levels of |
|
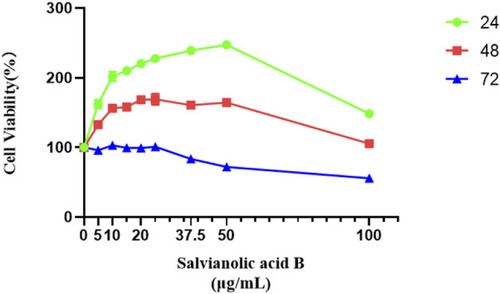
Salvianolic acid B promoted the proliferation of lymphatic endothelial cells. The image represents the viability of lymphatic endothelial cells treated with different dosages of Salvianolic acid B (5, 10, 15, 20, 25, 37.5, 50, and 100 μg/ml). The green curve represents the cell viability after 24 h of treatment with different concentrations of SAB. The red curve represents the cell viability after 48 h of treatment with different concentrations of SAB. The blue curve represents the cell viability after 72 h of treatment with different concentrations of SAB. |
|
Salvianolic acid B promoted the migration of lymphatic endothelial cells. The image represents the changes in the scratch width after Salvianolic acid B treatment. |
|
The protein expressions of VEGF-C and VEGFR-3. LECs were treated with different concentrations of SAB for 24h, and cell lysates were prepared and analyzed by western blot assay. |
|
Salvianolic acid B promoted the protein expressions of VEGF-C and VEGFR-3 proteins in lymphatic endothelial cells under injury. After the LECs were treated with MAZ51 for 24 h to induce injury, along with the treatment of three dosages of SAB for 24 h, cell lysates were prepared and analyzed by western blot assay. |