- Title
-
Mycobacteriophage-antibiotic therapy promotes enhanced clearance of drug-resistant Mycobacterium abscessus
- Authors
- Johansen, M.D., Alcaraz, M., Dedrick, R.M., Roquet-Banères, F., Hamela, C., Hatfull, G.F., Kremer, L.
- Source
- Full text @ Dis. Model. Mech.
|
|
|
|
|
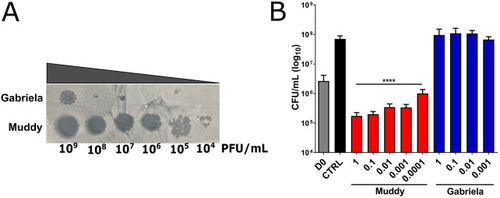
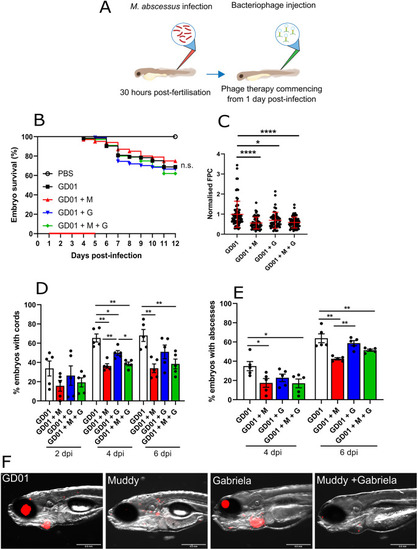
Impact of bacteriophage Muddy on GD01 infection in wild-type zebrafish. At 30 hpf, embryos were infected with 250-300 CFU of GD01 expressing tdTomato via caudal vein injection. At 1 dpi, embryos commenced phage therapy through caudal vein administration at an MOI of 50:1 (phage:bacteria) based on the initial bacterial infection inoculum. Embryos were treated daily from 1 dpi up to and including 5 dpi with either Muddy (M), Gabriela (G) or a phage cocktail containing both Muddy and Gabriela (M+G). (A) A generalised schematic showing the experimental design corresponding to the figure. (B) Embryo survival was monitored over a 12 day period, with embryos counted daily. Survival curves were analysed using the log-rank (Mantel–Cox) statistical test. The red bar across the x-axis indicates the duration of daily phage administration in the current experiment. (C) Bacterial burden [fluorescent pixel count (FPC)] was analysed at 6 dpi using fluorescent microscopy. Fluorescent images were analysed in ImageJ using the ‘Analyze particles’ function. Bacterial burden was analysed using a Kruskal–Wallis one-way ANOVA. (D,E) The proportion of embryos with cords (D) and abscesses (E) was enumerated at 2, 4 and 6 dpi using fluorescent microscopy. Abscess and cord quantification was analysed using a Kruskal–Wallis one-way ANOVA. Data shown are the mean of three independent experiments±s.d. (n=20-30 per group for each experiment). (F) Representative zebrafish images of untreated (GD01) or phage-treated embryos at 6 dpi, showing the presence of extracellular bacterial cords. Red overlay represents GD01 expressing TdTomato. Scale bars: 0.5 mm. n.s., not significant; *P<0.05; **P<0.01; ****P<0.0001. |
|
PHENOTYPE:
|
|
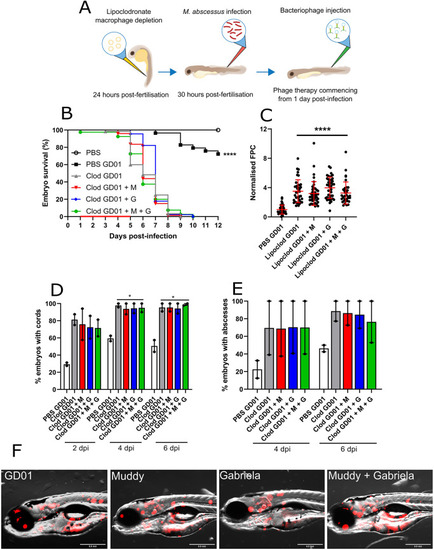
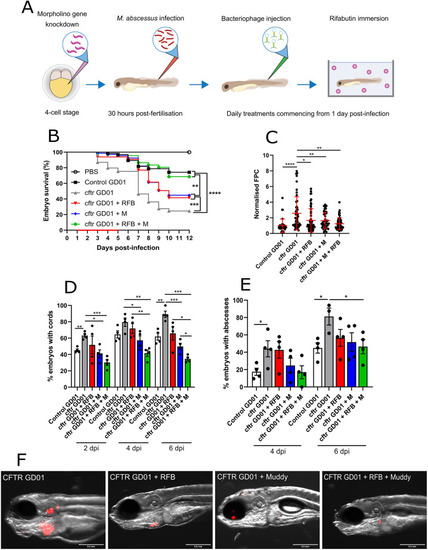
Effect of Muddy in GD01-infected cftr morphants. At the one- to four-cell stage, fertilised zebrafish eggs were injected in the nucleus with either control morpholino (control) or cftr morpholino (cftr). At 30 hpf, embryos were infected with 250-300 CFU of GD01 (control GD01 or cftr GD01) via caudal vein injection. At 1 dpi, embryos commenced phage therapy through caudal vein injection at an MOI of 50:1 (phage:bacteria) based on initial bacterial infection inoculum. Embryos were treated daily from 1 dpi, up to and including 5 dpi with Muddy (M), Gabriela (G) or a phage cocktail containing both Muddy and Gabriela (M+G). (A) A generalised schematic showing the experimental design corresponding to the figure. (B) Embryo survival was monitored over a 12 day period, with embryos counted daily. Survival curves were analysed using the log-rank (Mantel–Cox) statistical test. The red bar across the x-axis indicates the duration of daily phage administration in the current experiment. (C) Bacterial burden (FPC) was analysed at 6 dpi using fluorescent microscopy. Fluorescent images were analysed in ImageJ using the ‘Analyze particles’ function. Bacterial burden was analysed using a Kruskal–Wallis one-way ANOVA. (D,E) The proportion of embryos with cords (D) and abscesses (E) was enumerated at 2, 4 and 6 dpi using fluorescent microscopy. Abscess and cord quantification was analysed using a Kruskal–Wallis one-way ANOVA. Data shown are the mean of three independent experiments±s.d. (n=20-30 per group for each experiment). (F) Representative cftr morphant images of untreated (GD01) or phage-treated embryos at 6 dpi, showing the presence of extracellular bacterial cords or localised infection. Red overlay represents GD01 expressing TdTomato. Scale bars: 0.5 mm. *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001. PHENOTYPE:
|
|
PHENOTYPE:
|