- Title
-
Autophagy Blockade by Ai Du Qing Formula Promotes Chemosensitivity of Breast Cancer Stem Cells Via GRP78/β-Catenin/ABCG2 Axis
- Authors
- Liao, M., Wang, C., Yang, B., Huang, D., Zheng, Y., Wang, S., Wang, X., Zhang, J., Tang, C., Xu, Z., He, Y., Huang, R., Zhang, F., Wang, Z., Wang, N.
- Source
- Full text @ Front Pharmacol
|
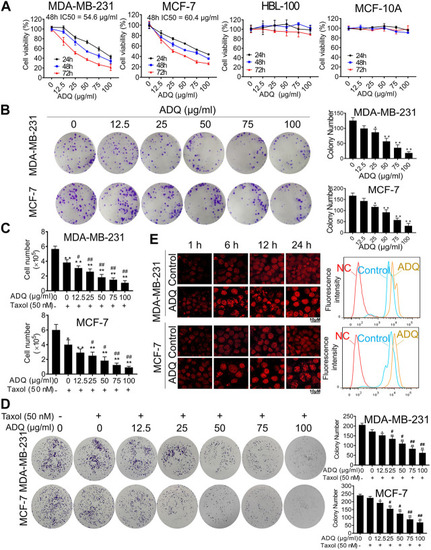
Ai Du Qing formula (ADQ) exerts anti-cancer and chemosensitivity effects on breast cancer cells. |
|
ADQ attenuates the proliferation, self-renewal and differentiation of breast CSCs. |
|
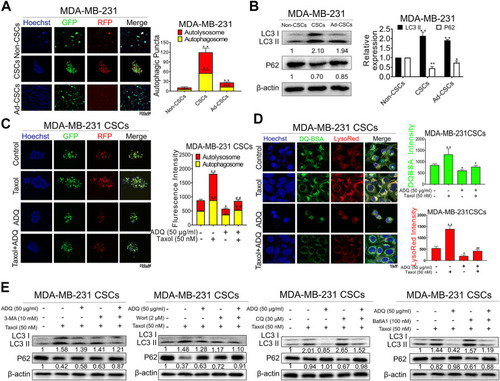
ADQ abrogates autophagy activity in breast CSCs. |
|
ADQ activates the Akt/GSK3β-mediated proteasome degradation of β-catenin in breast CSCs. |
|
GRP78 decreases breast cancer chemosensitivity possibly via autophagy induction of breast CSCs. |
|
GRP78 suppression by ADQ leads to a β-catenin destabilization and an autophagy inhibition in breast CSCs. |
|
ADQ enhances the chemosensitivity of breast cancer in |