- Title
-
Pathogenic role of delta 2 tubulin in bortezomib-induced peripheral neuropathy
- Authors
- Pero, M.E., Meregalli, C., Qu, X., Shin, G.J., Kumar, A., Shorey, M., Rolls, M.M., Tanji, K., Brannagan, T.H., Alberti, P., Fumagalli, G., Monza, L., Grueber, W.B., Cavaletti, G., Bartolini, F.
- Source
- Full text @ Proc. Natl. Acad. Sci. USA
|
Bort induces D2 levels in vivo. (A) Representative D2 and βIII IF staining of DRG cell bodies and the SN isolated from control rats or rats acutely treated with Bort (i.v. 0.2 mg/kg; 24 h). (B and C) Relative tubulin PTM levels measured by quantitative IF in randomly selected DRG cell bodies (35–60) and SN (five sections per condition) from rats treated with acute (n = 3 to 5 per group) (B) (i.v. 0.2 mg/kg; 24 h) or chronic (n = 4 per group) (C) (0.2 mg/kg, 3× week for 8 wk) doses of Bort. (D) Representative peripherin (smaller unmyelinated C-fibers), NF200 (medium and large myelinated A-β fibers), and D2 IF staining of DRG cell bodies acutely treated with Bort. (E) Relative D2 tubulin values measured in DRG cell bodies (n = 35 to 60) positive to either peripherin or NF200 from rats (n = 6) acutely treated with Bort (i.v. 0.2 mg/kg; 24 h). (F) Representative D2 and βIII IF staining of sural nerve biopsy from a patient with BIPN. (G) Ratio analysis of D2/βIII levels measured by IF of fixed tissue (three to six sections per condition) from one sural nerve biopsy of a BIPN patient versus three sural nerve biopsies from control patients. (H) Immunoblot analyses of D2 levels in whole cell lysates from one sural nerve biopsy from a patient treated with Bort displayed with a control patient. Tyr, tyrosinated tubulin. GADPH, loading control. All data in B, C, E, and G are shown as medians plus interquartile range, and statistics were analyzed by Mann–Whitney U test. *P < 0.05, **P < 0.01. Scale bars in A, D, and F, 20 μm. Red writings are P values when compared to control; black writings are P values when compared between groups. |
|
Bort induces D2 at the onset of axonal degeneration. (A) Representative IF images of D2 and βIII staining in axons of DRG neurons (12 DIV) treated with 100 nM of Bort for the indicated times. Scale bar, 50 μm. (B) Ratio analysis of D2/βIII levels measured by IF in axons from fixed neurons treated as in A. Data are pooled from three to four experiments (n = 10 to 41 neurites per condition for each experiment). (C) Time-dependent increase of axonopathy in DRG neurons treated with 100 nM of Bort for the indicated times. Data are from three to four experiments (n = 4 to 15 fields per condition for each experiment). (D) Immunoblot analyses of D2 levels from whole cell lysates of adult DRG neurons (12 DIV) treated with increasing doses of Bort for the indicated times. (E) Quantification of normalized D2 levels as in D. Data are pooled from five to six experiments. (F) Immunoblot analyses of D2 and βIII levels present in the MT pellet (P) and soluble tubulin (S) fractions isolated from adult DRG neurons (12 DIV) and treated with 100 nM of Bort for the indicated times. (G) Quantification of D2 and βIII levels as in F. Data are from four to eight experiments. All data in B, C, E, and G are shown as medians plus interquartile range, and statistics were analyzed by Mann–Whitney U test. *P < 0.05, **P < 0.01. |
|
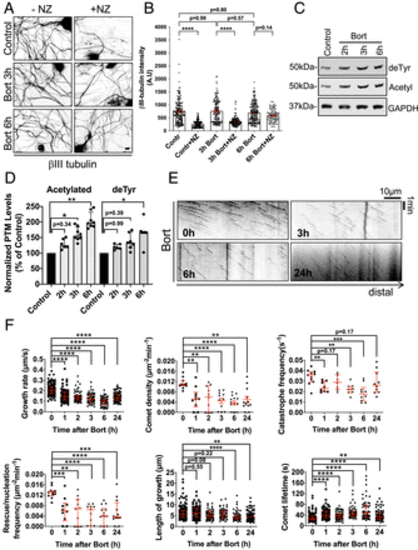
Bort acutely induces MT stability and affects MT behavior in DRG neurons. (A) Representative IF images of βIII in adult DRG neurons (12 DIV), treated with 100 nM of Bort for 3 and 6 h, and incubated with 1 μg/mL nocodazole (NZ) for 45 min before MT extraction and fixation. Scale bar, 10 μm. (B) Quantification of residual MT mass in proximal axons (100-μm from cell bodies; 50 to 150 axons per time point) of DRG neurons treated as in A. All data are means ± SEM and analyzed by ANOVA (multiple comparison). (C) Immunoblot analyses of de-tyrosinated (deTyr) and acetylated (Acetyl) tubulin levels in cultured 12 DIV treated with 100 nM of Bort for 2 to 6 h. (D) Quantification of normalized deTyr and Acetyl tubulin levels as in C. Data are from 6 to 8 experiments. (E) Representative kymographs at 0, 3, 6, and 24 h of EB3-GFP comets in DRG axons treated with 100 nM of Bort. Scale bar, 10 μm. (F) An EGFP-EB3 time course analysis of MT dynamics parameters in DRG neurons (12 DIV), treated as in E for the indicated times. Data were from up to 118 comets and 7 to 11 neurites for each group. Data in D and F are shown as medians plus interquartile range, and statistics were analyzed by Mann–Whitney U test. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. |
|
Bort acutely affects MT behavior in DRG neurons in vivo. (A) Still images from live imaging of mCherry in the peripheral nerve fibers of zebrafish treated with 1.3 μM Bort for the indicated times. Each image includes endings innervating the surface of a scale. Scale bar, 100 μm. (B) Representative kymographs at 0 and 6 h of EB3-GFP comets in the peripheral nerve fibers of zebrafish treated with Bort. (C) EB3-GFP time course analysis of MT dynamics parameters in the peripheral nerve fibers of zebrafish treated as in B for the indicated times. Data were pooled from three individual fish containing four to five neurites with up to 80 to 220 comets. (D) Micrographs from third-instar Drosophila larvae fed with vehicle control (n = 7 animals, n = 28 cells) or 20 μM Bort (n = 7 animals, n = 45 cells) starting 24 to 28 h after egg laying. ppk-CD4-tdGFP was used to visualize ddaC nociceptive neurons. (E) Quantification of dendrite degeneration treated as in D. Each point refers to an averaged value of degeneration index in each animal (4 to 6 cells per animal). Bars, 50 µm. (F) Representative kymographs of EB1-GFP comets in control and nociceptive neurons of Drosophila larvae fed with Bort for 3 and 6 h. Scale bar, 10 μm. (G) EB1-GFP time course analysis of MT dynamics parameters in nociceptive neurons of larvae treated for the indicated times. Data are from up to 400 comets and 19 to 45 neurites for each group. Control data are pooled from vehicle controls (3 to 6 h). Data in C, E, and G are medians plus interquartile range, and statistics were analyzed by Mann–Whitney U test. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. |
|
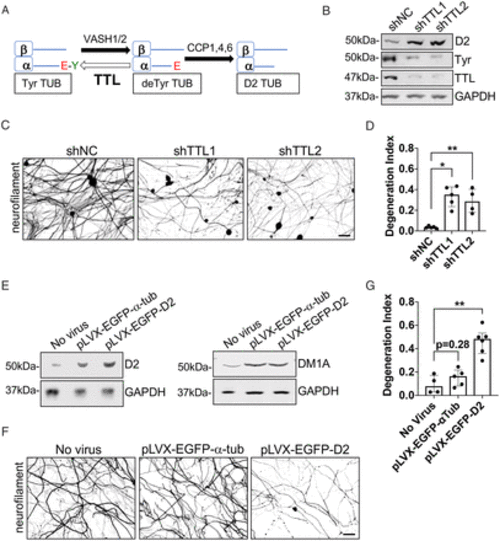
D2 accumulation is sufficient to drive Bort-induced axonopathy. (A) Schematic of the enzymes involved in D2 generation. (B) Immunoblot analyses of TTL, D2, and tyrosinated tubulin levels in adult DRG neurons (12 DIV) silenced of TTL expression by lentiviral shRNA delivery (5 DIV). (C) Representative IF images of neurofilament staining in DRG neurons (12 DIV) silenced of TTL expression for 7 d by lentiviral shRNA delivery. (D) Axonal degeneration in DRG neurons treated as in C. Data are pooled from 4 to 5 experiments (6 to 16 fields per condition for each experiment). (E) Immunoblot analyses of D2 and α-tubulin (DM1A) levels in adult DRG neurons (12 DIV) expressing D2-(pLVX-EGFP- D2) or WT α-tubulin (pLVX-EGFP-α−tub) (5 DIV). GAPDH, loading control. (F) Representative IF images of neurofilament staining in DRG neurons (12 DIV) transduced with lentiviruses expressing D2-(pLVX-EGFP-D2) or WT α-tubulin (pLVX-EGFP-α-tub) for 7 d. (G) Axonal degeneration in DRG neurons treated as in F. All data in D and G are shown as medians plus interquartile range from 4 to 6 experiments (4 to 17 fields per condition for each experiment). Statistical significance was analyzed by Mann–Whitney U test. GAPDH, loading control. shNC, shRNA noncoding control. *P < 0.05, **P < 0.01. Scale bars in C and F, 50 μm. |
|
D2 accumulation is necessary to drive Bort-induced axonopathy. (A) Representative IF images of neurofilament staining in DRG neurons silenced of CCP1 expression at 5 DIV and treated at 12 DIV with 100 nM of Bort for 24 h. Scale bar, 50 μm. (B) Axonal degeneration in DRG neurons treated as in A. Data are from at least 6 to 12 fields per condition (n = 4 experiments). Data are shown as medians plus interquartile range, and statistics were analyzed by Mann–Whitney U test. *P < 0.05. |
|
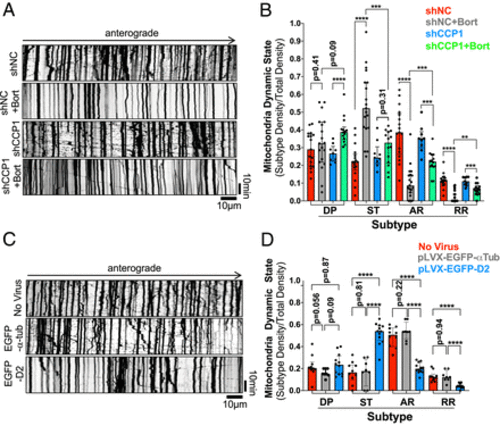
D2 accumulation is sufficient and necessary to drive defects in mitochondria motility caused by Bort. (A and C) Representative kymographs of mitochondria motility in DRG neurons infected with shCCP1 and shNC lentivirus (A) or pLVX-EGFP-D2 and WT-α-tubulin lentivirus (C) prior to infection with Mito-DsRed lentivirus and Bort treatment (A). Videos, 10 s/frame for 30 min. (B and D) Quantification of mitochondria dynamic state density in neurons treated as in A and C and expressed as a fraction of total mitochondria density. Dynamic Pause (DP); Stationary (ST); Anterograde Running (AR); Retrograde Running (RR). Data shown in B and D are from 9 to 19 (B) or 8 to 12 neurites (B) pooled from three independent experiments. All data in B and D are shown as medians plus interquartile range, and statistics were analyzed by Mann–Whitney U test. **P < 0.01, ***P < 0.001, and ****P < 0.0001. |