- Title
-
Propofol impairs specification of retinal cell types in zebrafish by inhibiting Zisp-mediated Noggin-1 palmitoylation and trafficking
- Authors
- Fan, X., Yang, H., Hu, L., Wang, D., Wang, R., Hao, A., Chen, X.
- Source
- Full text @ Stem Cell Res. Ther.
|
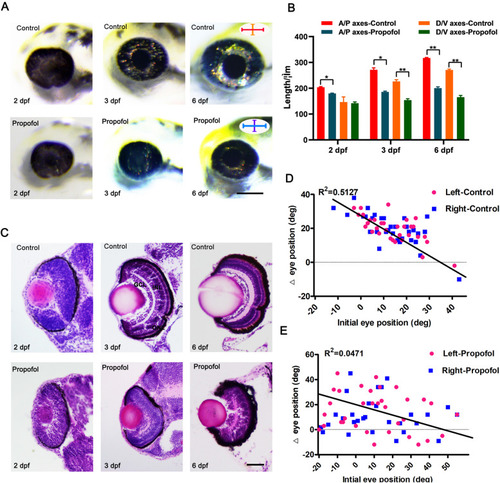
Propofol treatment results in microphthalmia and defects in retinal lamination and function. |
|
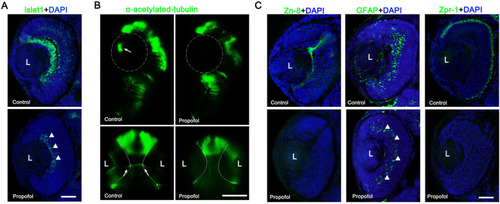
Propofol impairs the differentiation of retinal neurons and glia. Immunohistochemical analysis of markers (green) of neuronal and glial differentiation at 3 d postfertilization (dpf) ( |
|
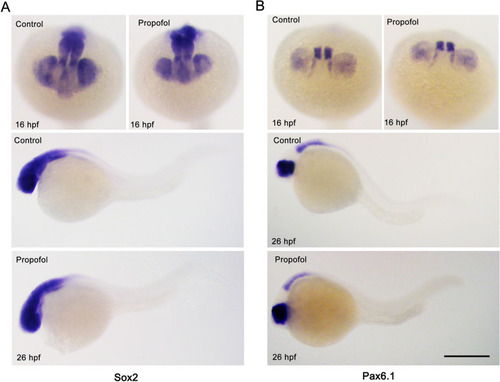
Propofol does not affect the specification of retinal progenitor cell identity. |
|
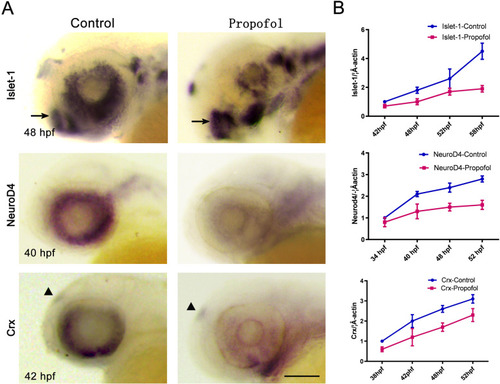
Propofol impairs the specification of retinal cell types. |
|
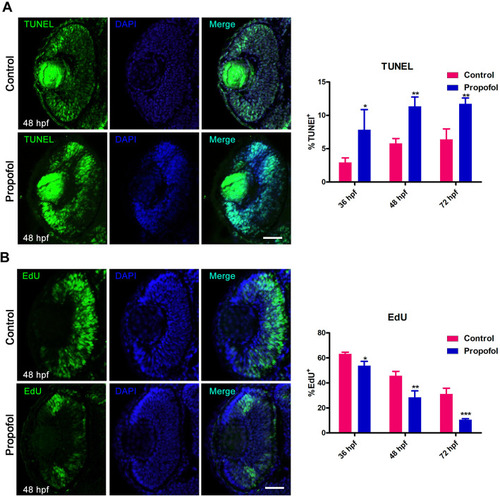
Propofol increases cell death and decreases the number of S-phase cells in retinas. |
|
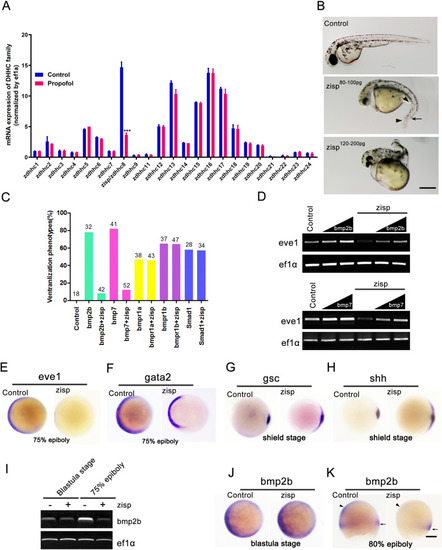
Zisp overexpression mimics noggin overexpression activity and acts upstream of the bone morphogenetic protein (BMP) receptor. |
|
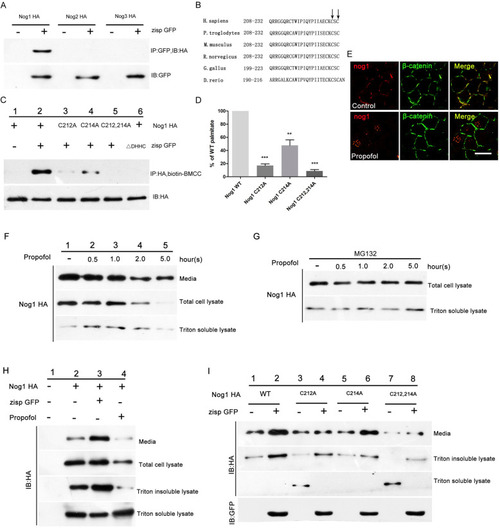
Palmitoylation increases the secretion and activity of Noggin-1. |