- Title
-
Fundc1 is necessary for proper body axis formation during embryogenesis in zebrafish
- Authors
- Xu, G., Shen, H., Nibona, E., Wu, K., Ke, X., Al Hafiz, M.A., Liang, X., Zhong, X., Zhou, Q., Qi, C., Zhao, H.
- Source
- Full text @ Sci. Rep.

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |
|
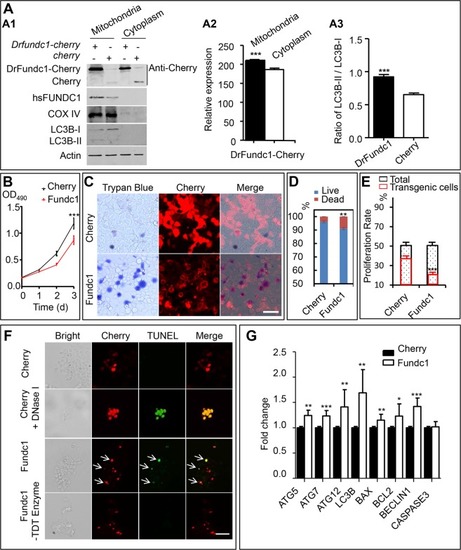
DrFundc1 reduced cell viability while inducing autophagy and apoptosis in transgenic 293 T cells. ( |
|
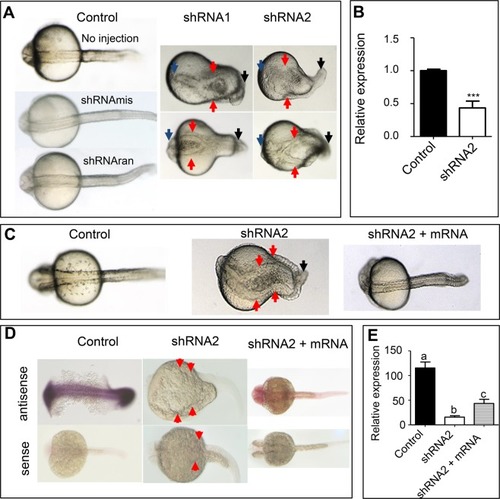
Knockdown of |
|
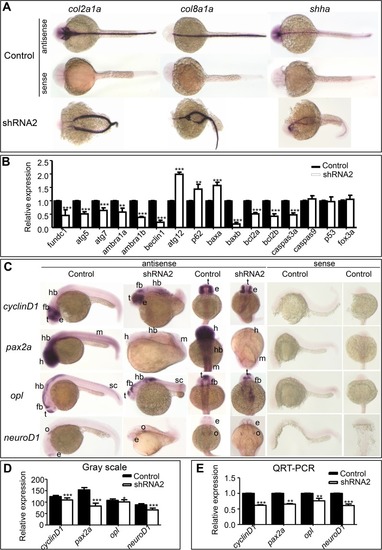
Expression of genes related to body axis formation, autophagy, and apoptosis. ( |