- Title
-
Paralogues of Mmp11 and Timp4 Interact during the Development of the Myotendinous Junction in the Zebrafish Embryo
- Authors
- Matchett, E.F., Wang, S., Crawford, B.D.
- Source
- Full text @ J Dev Biol
|
Sequences and predicted structures of zebrafish metalloproteinase 11 (Mmp11) and tissue inhibitors of metalloproteinase-4 (Timp4) paralogues exhibit both conserved and divergent features. ( |
|
Schematic illustration of the construction of EMMA–Mmp11a. The coding sequence of zebrafish |
|
Mmp11a is activated by furin except in the nucleus. ( |
|
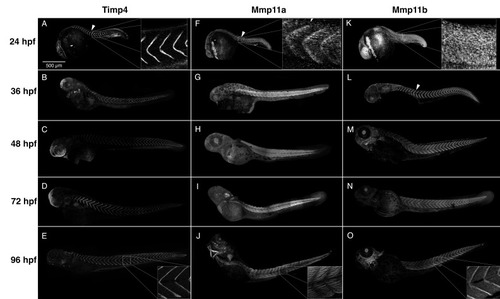
Timp4 and Mmp11 paralogues accumulate dynamically during development and co-localize at the myotendinous junctions (MTJs). Composite confocal projections of 24, 36, 48, 72 and 96 hpf embryos labeled with antibodies against Timp4, Mmp11a, or Mmp11b. ( |
|
Timp4 and both Mmp11 paralogues are present in the MTJ at 28 hpf. High-resolution confocal micrographs of MTJs in the trunk skeletal musculature dorsal to the yolk extension of 28 hpf embryos stained with antibodies against ( EXPRESSION / LABELING:
|
|
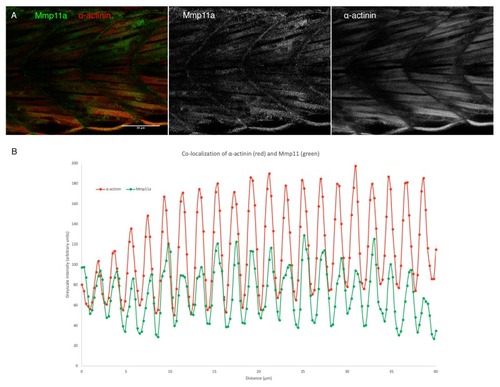
Mmp11a localizes to the Z-discs of skeletal muscle sarcomeres. ( EXPRESSION / LABELING:
|
|
Schematic representation of hypothesized activities and interactions between Mmp11 and Timp4 paralogues at early and late MTJs. The hemopexin-like domain of Mmp11a interacts with the C-terminal domain of Timp4b in the early MTJ, facilitating the degradation of fibronectin. In the mature MTJ, Mmp11b is inhibited by Timp4a, constraining its activity in the maintenance of the ECM, while Mmp11a localizes to the Z-discs of the sarcomeres within the myocytes. |