- Title
-
Polyacrylamide Bead Sensors for in vivo Quantification of Cell-Scale Stress in Zebrafish Development
- Authors
- Träber, N., Uhlmann, K., Girardo, S., Kesavan, G., Wagner, K., Friedrichs, J., Goswami, R., Bai, K., Brand, M., Werner, C., Balzani, D., Guck, J.
- Source
- Full text @ Sci. Rep.
|
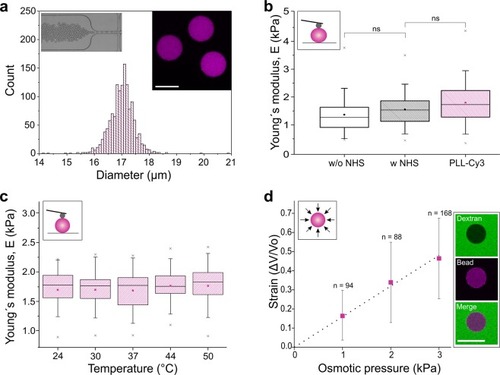
Mechanical characterization of polyacrylamide (PAAm) beads. ( |
|
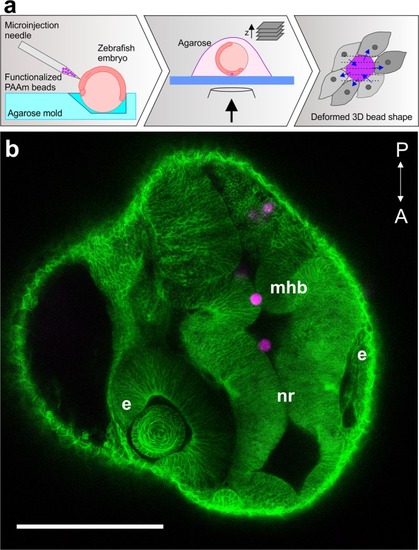
PAAm bead microinjection into zebrafish embryos. ( |
|
|
|
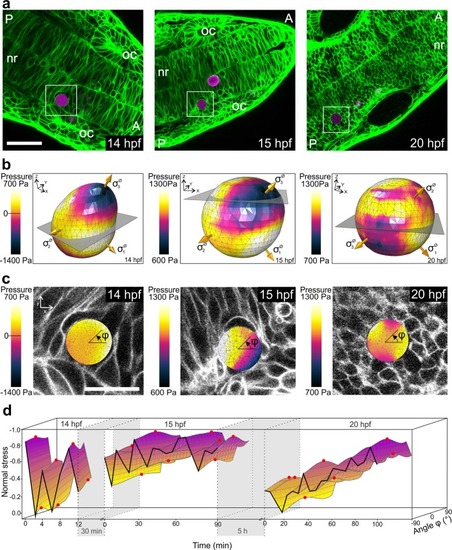
Spatial and temporal normal stress variations within the zebrafish neural rod during development. ( |