- Title
-
Method for disarranging the pigment pattern of zebrafish by optogenetics
- Authors
- Aramaki, T., Kondo, S.
- Source
- Full text @ Dev. Biol.
|
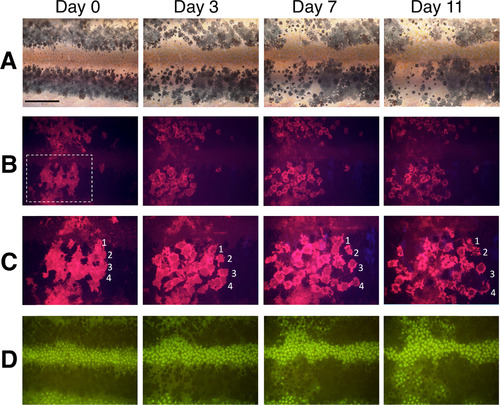
BL stimulation activates melanophore migration in vivo. (A–D) Serial images showing sequential changes in the pigment pattern of mitfa;ChR2(C128S/D156A) F0 transgenic fish during ongoing BL irradiation. Fish were reared in the dark until 5 wpf and then exposed to BL. (A) Bright-field images of melanophore stripes with ChR2(C128S/D156A)-mCherry transgene expression. (B) Red fluorescence images showing the shape of transgenic melanophores in the left half of the lower black stripe, highlighted with a white dashed box. (C) Magnified images of the area in the dashed square shown in panel B. Four different melanophores are indicated as 1–4. (D) Xanthophores were visible under green autofluorescence. Scale bar, 500 µm. Magnified and detailed images of Fig. 2 are shown in Supplementary Fig. S3. |
|
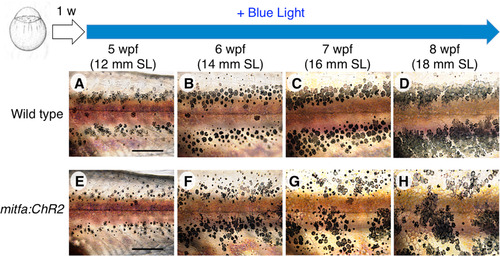
Hypermotility of melanophores perturbs stripe boundaries and disrupts the horizontal pattern. Sequential images show the process of skin pattern formation. Fish were reared under BL from the larval stage (1 wpf). (A–D) In wild-type fish, stripe boundaries became increasingly distinct across development; the initial horizontal stripe pattern was firmly maintained. (E–H) In mitfa;ChR2(C128S/D156A) stable transgenic fish, the initial horizontal pattern formed at 5 wpf, as in wild type, but stripe boundaries remained ambiguous even at later stages, and the stripes themselves were disrupted. Scale bar, 500 µm. |
|
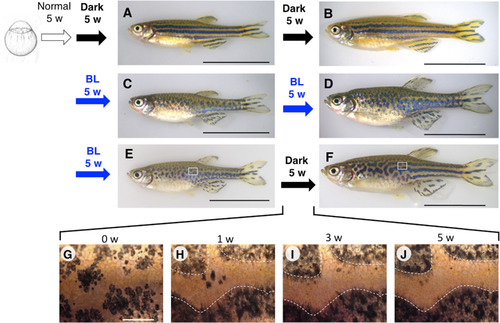
BL-induced migration of melanophores allows temporal disruption of pattern formation. mitfa:ChR2(C128S/D156A) stable transgenic fish were reared under different light conditions. Fish were maintained under normal light until 5 wpf. (A, B) Transgenic fish continuously maintained in the dark starting from 5 wpf showed a near-normal stripe pattern at 10 wpf (A, 17.5 mm SL, number of samples: n = 7) and 15 wpf (B, 21.4 mm SL, n = 3). (C, D) Transgenic fish continuously exposed to BL starting from 5 wpf showed a disrupted stripe pattern at 10 wpf (C, 17.5 mm SL, n = 3) and 15 wpf (D, 22.5 mm SL, n = 12). (E, F) Transgenic fish exposed to BL from 5 to 10 wpf and then transferred to the dark showed a disrupted stripe pattern at 10 wpf (E, 19.4 mm SL, n = 6); a pattern was regenerated at 15 wpf, but the stripes in the trunk mostly lacked horizontal directionality, especially anteriorly, whereas those in the tail regained a pattern highly reminiscent of that of the WT (F, 23.2 mm SL, n = 3). (G–J) Magnified images showing the time course of pattern regeneration in the dark; ambiguous stripe boundaries became distinct after 5 weeks. Numbers above each panel indicate weeks elapsed after transfer to the dark condition. White dashed lines indicate boundaries between melanophore and xanthophore regions at 5 weeks after transfer to the dark (J). The boundaries were overlaid on images of 1 week and 3 weeks after transfer (H, I). The pattern disruption/regeneration experiments were perfumed more than three times. Black scale bar, 10 mm; white scale bar, 500 µm. |
|
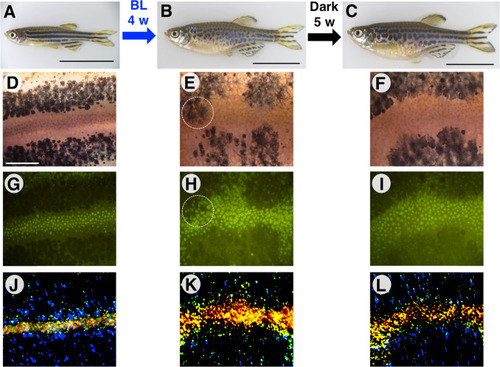
Forced melanophore migration resets the established stripe pattern. (A) mitfa:ChR2(C128S/D156A) stable transgenic fish were reared in the dark until 6.5 wpf. A well-established stripe pattern is shown. 16.5 mm SL. (B) The same transgenic fish were irradiated with BL for 4 weeks, disrupting the stripe pattern. 24.0 mm SL. (C) Five weeks after the fish was returned to the dark, a stripe pattern regenerated that was partly lacking horizontal directionality. 25.0 mm SL. (D–F) Bright-field images of melanophores. (G–I) Green autofluorescence of xanthophores in fluorescence images. (J–L) Light reflection from iridophores in dark-field images. Yellow indicates dense iridophores and blue indicates loose, dispersed iridophores. The three pigment cell types were rearranged after BL irradiation. Black scale bar, 10 mm; white scale bar, 500 µm. Magnified images of the regions surrounded by white-dashed lines are shown in Supplementary Fig. S6. |
Reprinted from Developmental Biology, 460(1), Aramaki, T., Kondo, S., Method for disarranging the pigment pattern of zebrafish by optogenetics, 12-19, Copyright (2018) with permission from Elsevier. Full text @ Dev. Biol.