- Title
-
Performing DNA nanotechnology operations on a zebrafish
- Authors
- Yang, J., Meng, Z., Liu, Q., Shimada, Y., Olsthoorn, R.C.L., Spaink, H.P., Herrmann, A., Kros, A.
- Source
- Full text @ Chem Sci
|
| Fluorescent labeling and DNA replacement on the surface of zebrafish embryos. (a) Lipid DNA (U4T18) is anchored on the skin membrane of 1 dpf zebrafish embryos, and (b) hybridizes to 1 μM ATTO594 fluorescently labeled complementary DNA (C594) (step 1), resulting in red fluorescence on the zebrafish surface. (c) A 20-mer oligonucleotide replaces C594 by means of strand displacement (step 2), resulting in loss of fluorescence of the fish. (d) Addition of 1 μM ATTO488 fluorescently labeled complementary DNA (C488) results in hybridization with U4T18 (step 3) and the appearance of a green fluorescence at the zebrafish surface. The insert represents the sequences of C594, 20-mer and C488. Red channel = ATTO594; green channel = ATTO488. |
|
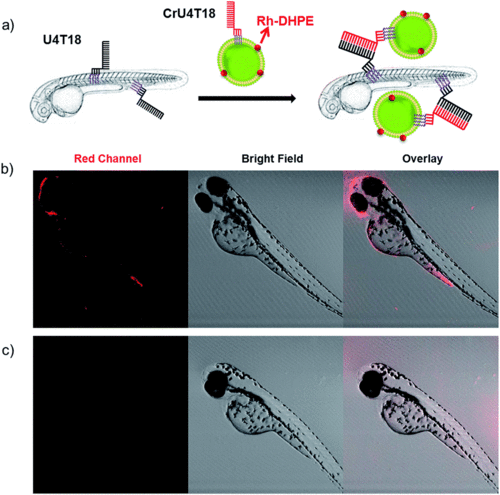
DNA duplex formation between U4T18 and CrU4T18 decorated liposomes on the surface of zebrafish. (a) Schematic representation of liposomes docking on the surface of zebrafish embryos by lipid–DNA hybridization. Confocal images of 2 dpf zebrafish treated with (b) U4T18 for 1 h, followed by incubation with CrU4T18 decorated liposomes or (c) treated with 1 μM CrU4T18 decorated liposomes in absence of U4T18. The concentration of total lipids (DOPC |
|
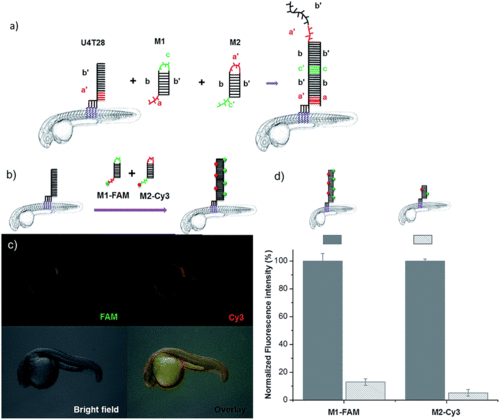
DNA hybridization chain reaction (HCR) on the surface of zebrafish embryos. (a) a′, b′, and c′ are regions that are complementary to regions a, b, and c, respectively. Hairpin M1 can be unfolded by hybridization with initiator U4T28, resulting in growing DNA strands. (b) Addition of 6-FAM fluorescently labeled M1 (M1-FAM) and Cy3 fluorescently labeled M2 (M2-Cy3) to the U4T28 pre-treated zebrafish results in DNA HCR and concomitant increase in fluorescence. (c) Fluorescence images of 1 dpf zebrafish embryos after incubation with U4T28 for 1 h and subsequent exposure to 1 μM M1-FAM and M2-Cy3 for 1 h. Green channel: 6-FAM; red channel: Cy3. (d) Normalized fluorescence intensity of attached DNA on the surface of zebrafish embryos. Fluorescence intensities of images (c) and ESI Fig. S3a† were calculated by Image J and plotted as a percentage relative to the fluorescence of M1-FAM or M2-Cy3 of Fig. 3c. The intensities of Fig. 3c were set to 100%. |
|
In vivo DNA HCR enhances the fluorescence intensity of labeling. (a) Fluorescence images of 2 dpf zebrafish embryos that were first decorated with U4T28, followed by 3 times washing with egg water, incubation with M1-FAM for 1 h and M2 for another 1 h. (b) Only FAM fluorescent labeled M1 (M1-FAM) was added after anchoring of U4T28 on the fish and washing three times with egg water. Green channel: M1-FAM. |


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: