- Title
-
Adipocytes Promote Early Steps of Breast Cancer Cell Dissemination via Interleukin-8.
- Authors
- Vazquez Rodriguez, G., Abrahamsson, A., Jensen, L.D.E., Dabrosin, C.
- Source
- Full text @ Front Immunol
|
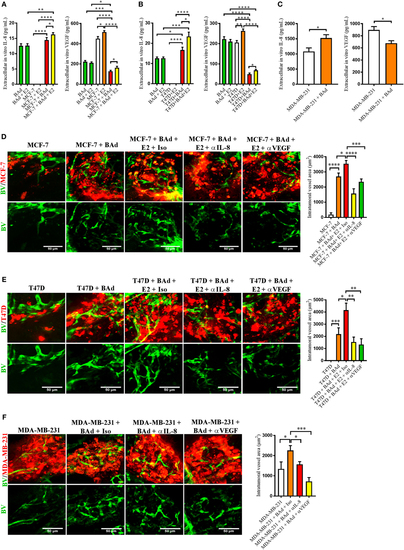
Addition of breast adipocytes (BAd) significantly increased interleukin-8 (IL-8) secretion but decreased vascular endothelial growth factor (VEGF) secretion compared to breast cancer cells (BCC) cultured alone and anti-IL-8 and anti-VEGF significantly decreased BAd-induced angiogenesis in primary tumors in zebrafish. BCC were cultured in 3D spheres alone or in combination with BAd. Secreted IL-8 and VEGF were analyzed as described in Section “Materials and Methods.” Prior injections, breast pre-adipocytes were differentiated for 12 days and estrogen receptor positive (ER+) BCC were cultured ± β-estradiol (E2) 1 nM for 48 h. All BCC were labeled with 4 µg/ml Fast DiI™ oil red dye. Cells were injected ± anti-IL-8, anti-VEGF, or isotype control at 0.1 mg/ml ± E2 1 nM into the perivitelline space of 2 days old zebrafish embryos, which expressed enhanced green fluorescent protein in endothelial cells. (A) BAd mammospheres alone and low metastatic ER+ MCF-7 ± 90% BAd mammospheres were cultured ± E2 1 nM during 7 days, n = 4–5 in each group. (B) BAd mammospheres alone an ER+ T47D with intrinsically higher metastatic capacity ± 90% BAd mammospheres were cultured ± E2 1 nM during 7 days, n = 5–6 in each group. (C) Estrogen receptor negative (ER−) metastatic MDA-MB-23 ± 90% BAd and BAd mammospheres were cultured during 7 days, n = 4–5 in each group. (D) MCF-7 cells were injected alone or in combination with 50% BAd ± E2 1 nM, tumor angiogenesis was analyzed 3 days post-injections, n = 12–18 in each group. (E) T47D cells were injected alone or in combination with 50% Bad ± E2 1 nM, tumor angiogenesis was analyzed 3 days post-injections, n = 10 in each group. (F) MDA-MB-231 cells were injected alone or in combination with 50% BAd, tumor angiogenesis was analyzed 3 days post-injections, n = 7–10 in each group. Representative confocal images are shown for each cell line. BV = blood vessels. Results are presented as mean ± SEM and analyzed by Student’s t-test, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Data are representative of at least two independent experiments. |
|
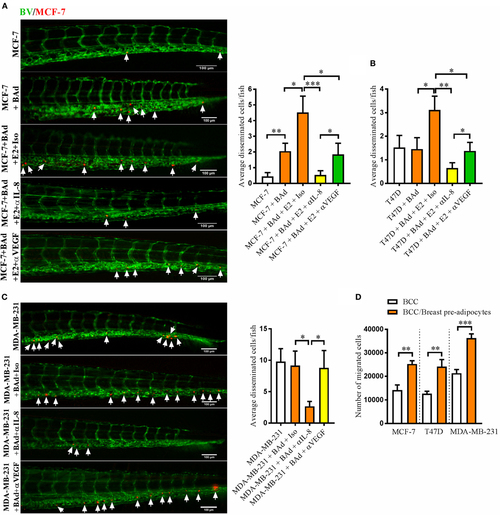
Anti-interleukin-8 (IL-8) treatment significantly decreased breast cancer cells (BCC) dissemination induced by breast adipocytes (BAd). Prior injections, breast pre-adipocytes were differentiated for 12 days and estrogen receptor positive (ER+) BCC were cultured ± β-estradiol (E2) 1 nM for 48 h. All BCC were labeled with 4 µg/ml Fast DiI™ oil red dye. Cells were injected ± anti-IL-8, anti-VEGF, or isotype control at 0.1 mg/ml ± E2 1 nM into the perivitelline space of 2 days old zebrafish embryos, which expressed enhanced green fluorescent protein in endothelial cells. (A) MCF-7 cells were injected alone or in combination with 50% BAd ± E2 1 nM. BCC dissemination was evaluated 3 days post-injections, n = 18–27 in each group. (B) T47D cells were injected alone or in combination with 50% BAd ± E2 1 nM. BCC dissemination was evaluated 3 days post-injections, n = 16–28 in each group. (C) MDA-MB-231 cells were injected alone or in combination with 50% BAd. BCC dissemination was evaluated 3 days post-injections, n = 14–18 in each group. (D) MCF-7, T47D, and MDA-MB-231 cells were cultured alone or in combination with 50% breast pre-adipocytes in vitro during 24 h, and migration of cells was determined as described in Section “Materials and Methods,” n = 6 in each group. BV = blood vessels. Arrows indicate disseminated BCC. Results are presented as mean ± SEM and analyzed by Student’s t-test, *p < 0.05, **p < 0.01, ***p < 0.001. Data are representative of at least two independent experiments. |
|
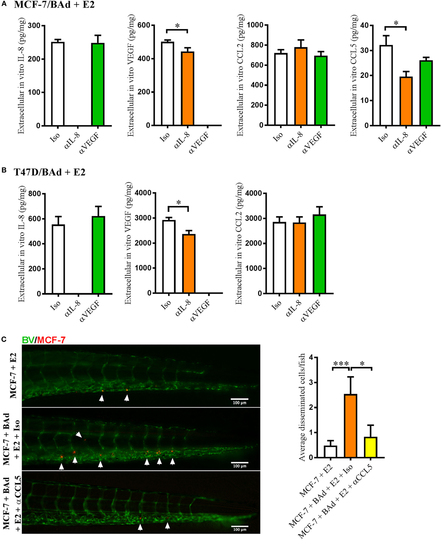
Anti-interleukin-8 (αIL-8) decreased vascular endothelial growth factor (VEGF) and CCL5 secretion, which affected estrogen receptor positive (ER+) breast cancer cells (BCC) dissemination. For monolayer co-cultures, breast pre-adipocytes were differentiated for 5 days before ER+ BCC were added at 4 × 103 cells/well. For zebrafish experiments, breast pre-adipocytes were differentiated for 12 days, MCF-7 cells were cultured + β-estradiol (E2) 1 nM for 48 h and labeled with 4 µg/ml Fast DiI™ oil red dye before injected into the perivitelline space of 2 days old zebrafish embryos, which expressed enhanced green fluorescent protein in endothelial cells. (A) MCF-7 cells were co-cultured with 50% breast adipocytes (BAd) in the presence or absence of αIL-8, anti-VEGF (αVEGF), or control isotype (Iso) antibodies at 1 µg/ml during 3 days in the presence of E2 1 nM, and secreted cytokines were quantified as described in Section “Materials and Methods,” n = 5–4 in each group. (B) T47D cells were co-cultured with 50% BAd in the presence or absence of αIL-8, αVEGF, or control Iso antibodies at 1 µg/ml during 3 days in the presence of E2 1 nM, and secreted cytokines were quantified as described in Section “Materials and Methods,” n = 6–5 in each group. (C) MCF-7 cells were injected in zebrafish embryos alone or in combination with 50% BAd ± anti-CCL5 (αCCL5) or Iso control antibody at 0.1 mg/ml and E2 1 nM, as described in Section “Materials and Methods.” MCF-7 dissemination was analyzed 3 days post-injections, n = 13–27 in each group. Representative images of zebrafish embryos are shown. Arrows show disseminated BCC cells. BV = blood vessels. Results are presented as mean ± SEM, Student’s t-test, *p < 0.05, ***p < 0.001. Data are representative of at least two independent experiments. |
|
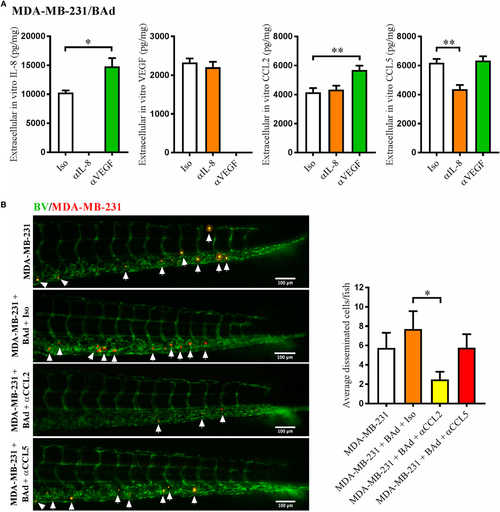
Anti-interleukin-8 (αIL-8) decreased CCL5 and anti-vascular endothelial growth factor (αVEGF) increased interleukin-8 (IL-8) and CCL2, which affected the dissemination of estrogen receptor negative (ER−) breast cancer cells (BCC). For monolayer co-cultures, breast pre-adipocytes were differentiated for 5 days before MDA-MB-231 cells were added at 3 × 103 cells/well. For zebrafish experiments, breast pre-adipocytes were differentiated for 12 days and MDA-MB-231 cells were labeled with 4 µg/ml Fast DiI™ oil red dye before injected into the perivitelline space of 2 days old zebrafish embryos, which expressed enhanced green fluorescent protein in endothelial cells. (A) MDA-MB-231 cells were co-cultured with 50% breast adipocytes (BAd) in the presence or absence of αIL-8, αVEGF, or control isotype (Iso) antibodies at 1 µg/ml during 3 days, and secreted cytokines were quantified as described in Section “Materials and Methods,” n = 4 in each group. (B) MDA-MB-231 cells were injected in zebrafish embryos alone or in combination with 50% BAd ± anti-CCL2 (αCCL2), anti-CCL5 (αCCL5), or control Iso antibodies at 0.1 mg/ml, as described in Section “Materials and Methods.” BCC dissemination was analyzed 3 days post-injections, n = 20–24 in each group. Representative images of zebrafish embryos are shown. Arrows show disseminated BCC. BV = blood vessels. Results are presented as mean ± SEM, Student’s t-test, *p < 0.05, **p < 0.01. Data are representative of at least two independent experiments. |
|
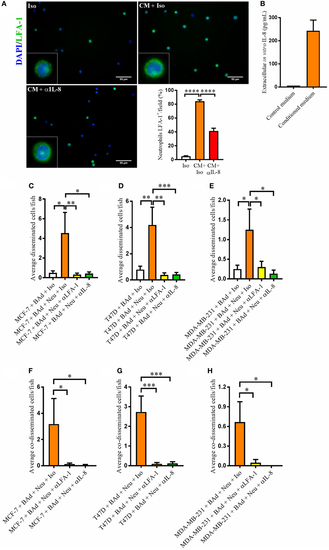
Anti-interleukin-8 (αIL-8) reduced lymphocyte function-associated antigen 1 (LFA-1) expression in neutrophils and the neutrophil-mediated dissemination of breast cancer cells (BCC) in the presence of breast adipocytes (BAd). For immunocytochemistry, neutrophils were cultured at 1 × 106 cells/ml in BAd-conditioned or control medium and incubated 45 min at 37°C. Prior zebrafish injections, breast pre-adipocytes were differentiated for 12 days, BCC were labeled with 4 µg/ml Fast DiI™ oil red dye, and neutrophils were labeled with 6 µg/ml DiB. All BCC were injected ± αIL-8, anti-LFA-1 (αLFA-1), or isotype (Iso) control antibodies at 0.1 mg/ml into the perivitelline space of 2 days old zebrafish embryos, which expressed enhanced green fluorescent protein in endothelial cells. (A) Neutrophils were cultured ± conditioned medium (CM) from BAd ± αIL-8 or Iso control at 1 µg/ml, and whole cells were stained with anti-human LFA-1 as described in Section “Materials and Methods” (n = 15 random fields per group). Insets show magnification of the cells. (B) Breast pre-adipocytes were differentiated during 12 days and cultured in DMEM supplemented medium during 24 h, and secreted interleukin-8 (IL-8) was measured in control and CM as described in Section “Materials and Methods,” n = 4 in the CM group. (C) MCF-7 cells were injected in zebrafish embryos together with 33% BAd ± 33% neutrophils, as described in Section “Materials and Methods.” BCC dissemination was analyzed at 1 day post-injections, n = 11–25 in each group. (D) T47D cells were injected in zebrafish embryos together with 33% BAd ± 33% neutrophils, as described in Section “Materials and Methods.” BCC dissemination was analyzed at 1 day post-injections, n = 15–26 in each group. (E) MDA-MB-231 cells were injected in zebrafish embryos together with 33% BAd ± 33% neutrophils, as described in Section “Materials and Methods.” BCC dissemination was analyzed at 1 day post-injections, n = 12–21 in each group. (F) Number of co-disseminated MCF-7/neutrophil cells was quantified as described in Section “Materials and Methods,” n = 17–21 in each group. (G) Number of co-disseminated T47D/neutrophil cells was quantified as described in Section “Materials and Methods,” n = 21–26 in each group. (H) Number of co-disseminated MDA-MB-231/neutrophil cells was quantified as described in Section “Materials and Methods,” n = 12–21 in each group. Results are presented as mean ± SEM, Student’s t-test, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Data are representative of at least two independent experiments. |
|
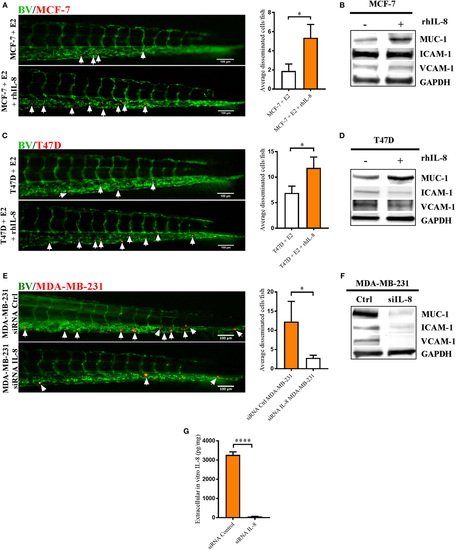
Interleukin-8 (IL-8) increased dissemination and MUC-1 expression in estrogen receptor positive (ER+) breast cancer cells (BCC) and IL-8 gene silencing affected the dissemination and expression of intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and mucin-1 (MUC-1) integrins in estrogen receptor negative (ER−) BCC. Prior injections, MCF-7 and T47D cells were cultured 48 h in β-estradiol (E2) 1 nM. All BCC were labeled with 4 µg/ml Fast DiI™ oil red dye and then injected into the perivitelline space of 2 days old zebrafish embryos, which express enhanced green fluorescent protein in endothelial cells. (A) MCF-7 cells were injected into zebrafish embryos ± recombinant human IL-8 (rhIL-8) at 1 µg/ml. BCC dissemination was analyzed after 3 days post-injections as described in Section “Materials and Methods,” n = 18–20 in each group. (B) Western blot analysis of MCF-7 cells treated ± rhIL-8 at 10 ng/ml during 5 days to evaluate the expression of MUC-1, VCAM-1, and ICAM-1. GAPDH load control was reused for illustrative purposes. (C) T47D cells were injected into zebrafish embryos ± rhIL-8 at 1 µg/ml. BCC dissemination was analyzed after 3 days post-injections as described in Section “Materials and Methods,” n = 17–23 in each group. (D) Western blot analysis of T47D cells treated ± rhIL-8 at 10 ng/ml during 3 days to evaluate the expression of MUC-1, VCAM-1, and ICAM-1. GAPDH is shown as load control. (E) MDA-MB-231 cells transfected with or without an IL-8 gene silencer RNA were injected into the perivitelline space of zebrafish embryos, and BCC dissemination was analyzed at 3 days post-injections, n = 19–22 in each group. (F) Western blot analysis of MDA-MB-231 cells transfected with/without an IL-8 silencer RNA (siIL-8) to evaluate the expression of MUC-1, VCAM-1, and ICAM-1. GAPDH load control was reused for illustrative purposes. (G) ELISA quantification of extracellular in vitro IL-8 in cell culture supernatants of MDA-MB-231 cells transfected with negative control silencer RNA (siRNA Control) or IL-8 silencer RNA (siRNA IL-8) during 2 days showed the successful knockdown of IL-8, n = 6 in each group. Representative images of zebrafish embryos are shown. Arrows show disseminated BCC. BV = blood vessels. Results are presented as mean ± SEM, Student’s t-test, *p < 0.05, ****p < 0.0001. Western blots shown in this figure were prepared by cropping and pasting from original membranes shown in full in Supplementary Figure 1. Data are representative of at least two independent experiments. |