- Title
-
Preclinical Animal Models for Dravet Syndrome: Seizure Phenotypes, Comorbidities and Drug Screening
- Authors
- Griffin, A., Hamling, K.R., Hong, S., Anvar, M., Lee, L.P., Baraban, S.C.
- Source
- Full text @ Front Pharmacol
|
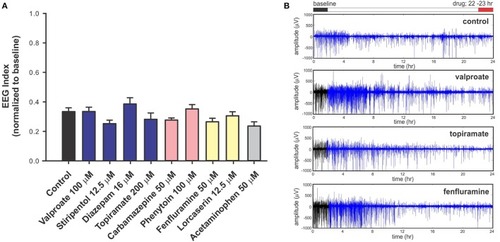
Electrophysiology analysis of |
|
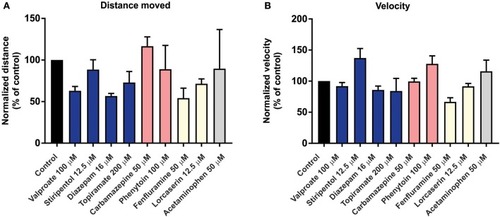
Behavior analysis of |
|
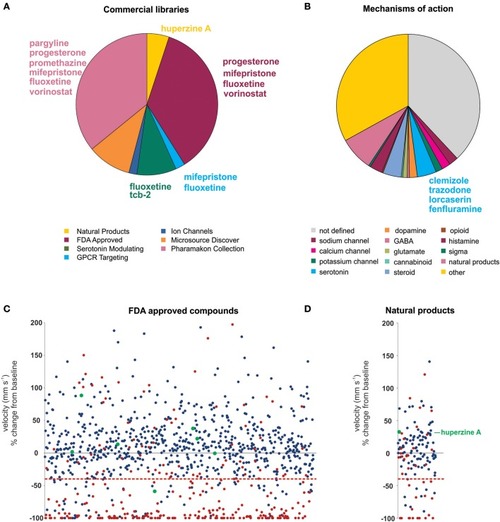
Summary of the compound screening results using the |
|
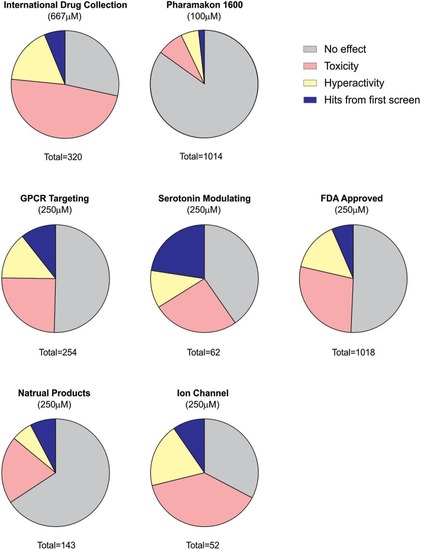
Summary of behavioral screening results for anti-seizure compounds in DS zebrafish larvae. In total, seven commercially available libraries have undergone blind screening for compounds which suppress the seizure activity in |