- Title
-
Acute stress is detrimental to heart regeneration in zebrafish
- Authors
- Sallin, P., Jaźwińska, A.
- Source
- Full text @ Open Biol.
|
In adult zebrafish, stress stimulates cortisol secretion and activates GCR signalling. (a) Adult zebrafish were exposed to different stressors. (b) Whole-body cortisol levels are shown as a dot plot. Each dot represents the value obtained for one fish. The dash indicates the mean value of each group (N e 10 fish; ***p < 0.001). H.S., heat shock; Caf, caffeine, Crow, crowding. (c) Live heart dissected from Tg(GCRE::EGFP) fish display GFP in the ventricle. (d,d2) Immunofluorescence analysis of Tg(GCRE::EGFP) heart section demonstrates a colocalization between tropomyosin and GFP. However, this transgenic reporter is not ubiquitously expressed in adult tissues. (e,f) Fluorescent images of the same transgenic animal Tg(GCRE::EGFP) reveal an enhanced glucocorticoid receptor (GCR) transcriptional activity in the whole body at 8 days after 1 h of daily crowding, as compared to the state before stress. (e2,f2) Converted images of the GFP signal into a grey scale using Image J software for quantification of fluorescence. (g) Graph showing the mean converted fluorescence values obtained for three different fish before and after the period with daily acute stress exposure. **p < 0.01. Scale bars: (c) 1 mm, (d) 100 µm and (e) 10 mm. |
|
Daily stress blocks cardiac regeneration after cryoinjury. (a-h) Heart sections at 30 dpci after AFOG staining showing the myocardium (orange), fibrin (red) and collagen (blue). The number in the upper right corner of each image represents the fraction of the analysed fish with the displayed phenotype. (i,j) Histograms represent the percentage of zebrafish hearts with complete (white), partial (grey) or blocked (black) regeneration. Numbers indicate days after cryoinjury for each experimental group. The regeneration scores were evaluated according to the area of collagen on multiple sections per each heart. N e 8; *p < 0.05, **p < 0.01, Fisher′s exact test. Scale bars, 100 µm. |
|
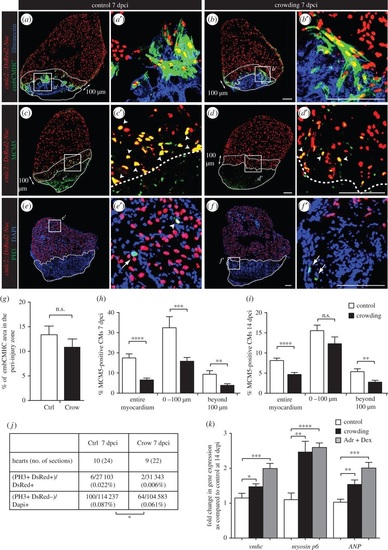
Daily acute stress exposure after cryoinjury affects CM proliferation and triggers compensatory mechanisms associated with cardiac overload. (a-f) Immunofluorescence staining of heart sections at 7 dpci of cmlc2::DsRed2-Nuc (cardiac nuclei, red) transgenic zebrafish of control and stressed animals. Dashed lines encircle the cryoinjured parts and a 100 µm-wide margin of the remaining myocardium along the injury border (peri-injury zone). (a-b′) No change in expression of embCMHC (embryonic isoform of cardiac myosin heavy chain, MAb N2.261, green) and fibronectin (blue) is observed between both experimental groups, N ≥ 4. (c-d′) The G1/S phase marker MCM5 (green) is reduced in CM nuclei of the stressed animals, particularly in the peri-injury zone. Arrowheads indicate double positive cells. (e-f′) The mitosis marker PH3 (green) is less frequent in the stressed animals as compared to controls. Arrows indicate mitotic non-CMs. (g-j) Quantification of immunofluorescence analysis shown in the representative images. N ≥ 8 hearts; three sections per heart. (k) qRT-PCR experiments revealed that exposure to crowding or dexamethasone/adrenaline (Adr + Dex) for 1 h d-1 during 14 days after cryoinjury results in upregulation of genes associated with cardiac compensatory response; N ≥ 2 sets, 10 hearts each. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Scale bars, 100 µm. EXPRESSION / LABELING:
|
|
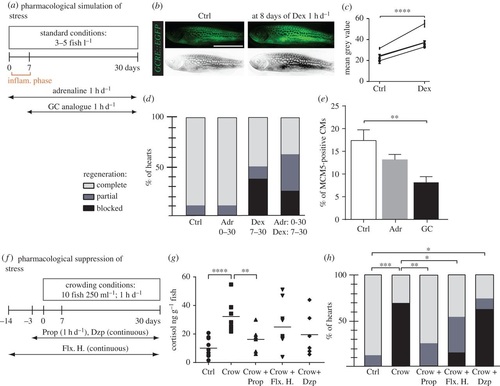
Pharmacological simulation of stress reproduces the crowding phenotype, which can be partially rescued by treatment with propranolol and fluoxetine hydrochloride. (a,f) Experimental design. (b) Live Tg(GCRE::EGFP) fish imaged for fluorescence (green converted to grey) before (Ctrl) and after 8 days of 2 mg l-1 dexamethasone (Dex) treatment for 1 h d-1 validates the activity of the drug. (c) Quantification of grey-converted fluorescence shown in (b); N = 4. (d,h) Regenerative scores of AFOG stained hearts at 30 dpci; N ≥ 8. (e) Quantification of MCM5 and DsRed-positive nuclei at 7 dpci in control and after treatments; N ≥ 6. (g) Measurements of whole-body cortisol after single stress exposure in fish with different pretreatment; N ≥ 5. Drugs: glucocorticoid (GC), dexamethasone (Dex, 2 mg l-1, 1 h d-1), adrenaline (Adr, 1 mg l-1, 1 h d-1), hydrocortisone (1 mg l-1), propranolol (Prop, 1 mg l-1, 1 h d-1), fluoxetine hydrochloride (Flx. H., 100 µg l-1, continuous), diazepam (Dzp, 1 mg l-1, continuous treatment). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. (b) Scale bar, 10 mm. |
|
RNA-seq analyses of control versus stressed animals identified three candidate genes, nr4a1, ankrd9 and igfbp1b, which are expressed in non-CMs of cryoinjured hearts. (a,d,g) Sections of hearts at 14 dpci immunostained against tropomyosin (TPM, green) and DAPI (blue). (b,e,h) The same sections as in (a,d,g) after in situ hybridization with RNA antisense probes. (c,f,i) Higher magnification of the framed area shown in (b,e,h) overlapped with tropomyosin (TPM, c′,f′,i′) immunostaining. (j,k) qRT-PCR experiments showed that hearts at 14 dpci exposed to stress or dexamethasone/adrenaline treatment for 1 h d-1 display a decrease in igfbp1b expression (N ≥ 3 sets of 10 cryoinjured ventricles). ***p < 0.001, ****p < 0.0001. Dashed lines encircle the cryoinjured areas. Scale bars, 100 µm. |
|
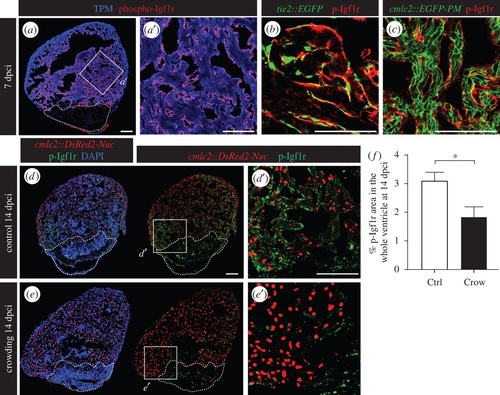
Daily stress decreases phospho-Igf1r activation in the regenerating heart. (a) Ventricular sections at 7 dpci reveal the presence of phospho-Igf1r (p-Igfr1, red) in the endocardium that covers cardiac muscle labelled by tropomyosin expression (TMP, blue). (b) p-Igf1r partially colocalizes with the pattern of endothelial cell layers demarcated by GFP expression in tie2::EGFP transgenic fish. (c) p-Igf1r does not overlap with the plasma membrane of CMs in cmlc2::EGFP-PM transgenic fish. (d,e) Sections of cmlc2::DsRed2-Nuc (red) hearts at 14 dpci display reduced p-Igf1r (green) expression in stressed animals. (f) Quantification of phospho-Igf1r area per ventricle at 14 dpci in control and stressed animals (N ≥ 7). *p < 0.05. Dashed lines encircle the cryoinjured areas. Scale bars, 100 µm. |
|
Inhibition of IGF signalling impairs CM proliferation. (a-d) Sections of cmlc2::DsRed2-Nuc (red) hearts at 7 dpci reveal that the treatment with 5 µM NVP-ADW742 (inhibitor of Igf1r) significantly reduces p-Igf1r levels (a,b, green) and MCM5-positive CMs (c,d, green). (e-f) Quantification of p-Igf1r (e, N ≥ 4) and the number of proliferating CMs (f, N ≥ 10) in the whole ventricles at 7 dpci. **p < 0.01, ***p < 0.001. Dashed lines encircle the cryoinjured areas. Scale bars, 100 µm. |