- Title
-
Multiple signaling factors and drugs alleviate neuronal death induced by expression of human and zebrafish tau proteins in vivo
- Authors
- Wu, B.K., Yuan, R.Y., Lien, H.W., Hung, C.C., Hwang, P.P., Chen, R.P., Chang, C.C., Liao, Y.F., Huang, C.J.
- Source
- Full text @ J. Biomed. Sci.
|
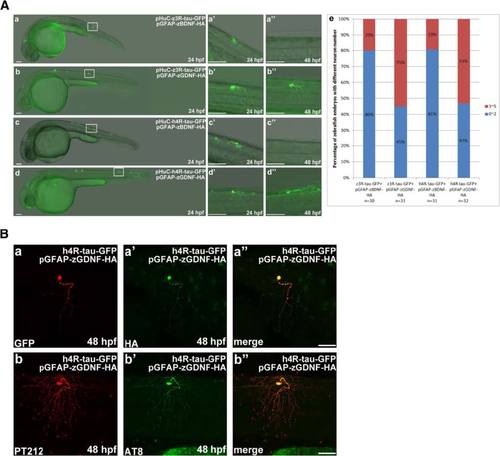
Overexpression of human and zebrafish tau proteins in zebrafish embryo resulted in neuronal death. a Schematic diagrams of each expression construct containing either wild-type or truncated forms of human and zebrafish Tau tagged with green fluorescence protein (GFP). Each expression construct was driven by the HuC promoter. The black bar represents one repeat of tubulin binding domain. The wild-type human and zebrafish tau proteins contain four and three repeats, respectively. b Each expression construct was microinjected into zebrafish embryos at the one-cell stage. Zebrafish embryos with GFP signals at 24 to 48 hpf were selected for image analysis. Embryos are shown in the lateral view with the anterior to the left and dorsal to the top. The boxed region of each panel (a-g) is enlarged (a′-g′′) to show the GFP-labeled neuronal cells in 24 to 48 hpf embryos from the lateral view. Scale bars: 100 µm. c The five GFP-labeled neuronal cells in embryos injected with pHuC-h4R-tau-GFP were traced with the aid of time-lapse recording. Puncta formation was observed in neuron E at 25 hpf, neuron C at 26 hpf, neuron B at 27 hpf, and neuron A at 28 hpf. Scale bars: 100 µm. d TUNEL staining (panel a) and double immunostaining of zebrafish embryos expressing h4R-tau-GFP at different developmental stages was performed using polyclonal antibody against Caspase 9 and monoclonal antibody against GFP (panel b). Scale bar: 50 µm |
|
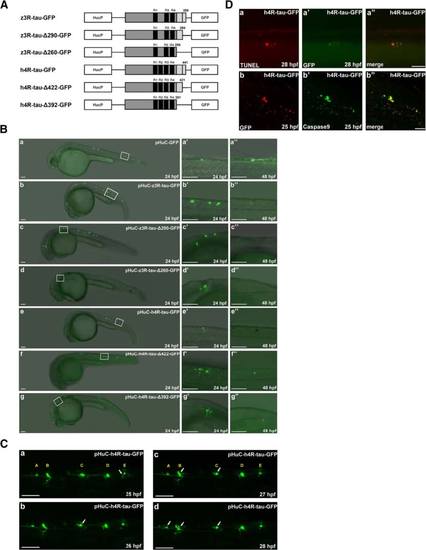
Zebrafish Bcl2-L1 overexpression prevented human 4R-tau-GFP and zebrafish 3R-tau-GFP induced Neuronal death. a GFP-labeled neuronal cells and axons were observed at 24 and 48 hpf in embryos co-injected with pHuC-zBcl2-L1-HA-2A-mCherry and pHuC-z3R-tau-GFP (b) or pHuC-h4R-tau-GFP (d). For comparison, embryos co-injected with pHuC-mCherry (panels a and c) were used as the control. The boxed regions are enlarged (a′-d′′) to show the GFP-labeled neuronal cells in 24 and 48 hpf embryos from the lateral view. Scale bars: 100 µm. The protection effect of zBcl2-L1 against neuronal death by human tau-GFP or zebrafish tau-GFP was presented in panel e to show higher percentage, 69 % and 66 % of zebrafish embryos expressing zBcl2-L1 with more neuronal cells, compared to 21 % and 18 % without zBcl2-L1. b GFP signals (panel a) and mCherry signals (panel a′) in neuronal cells and axons in embryos co-injected with pHuC-h4R-tau-GFP and pHuC-zBcl2-L1-HA-2A-mCherry were colocalized (panel a′′). Scale bar: 50 µm. c Double immunostaining of h4R-tau-GFP (GFP antibody, panel a) and Bcl2-L1-HA (HA antibody, panel a′) in spinal cord neurons of the aforementioned zebrafish embryos. The phosphorylation state of h4R-tau-GFP was detected using antibody pT212 (panel b) and antibody AT8 (panel b′). Embryos are shown from the lateral view with the anterior to the left and dorsal to the top. Scale bar: 50 µm. d Double immunostaining of zebrafish embryos expressing h4R-tau-GFP at different developmental stages was performed using polyclonal antibody against GFP and monoclonal antibody AT8. Scale bar: 50 µm. e Western blot analysis of total protein extract of zebrafish embryos expressing h4R-tau-GFP at 24 hpf was performed using polyclonal antibody against GFP and monoclonal antibody AT8 |
|
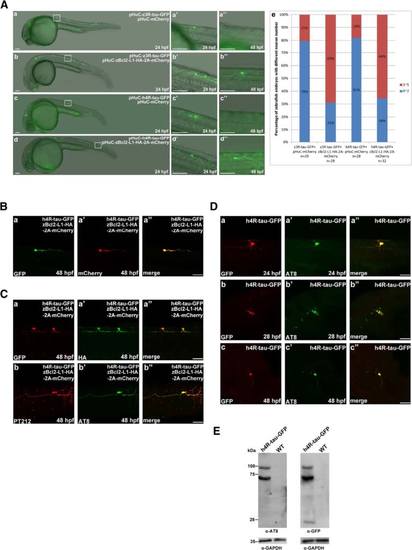
Zebrafish Nrf2 overexpression prevented human 4R-tau-GFP and zebrafish 3R-tau-GFP induced Neuronal death. a GFP-labeled neuronal cells and axons were observed at 24 and 48 hpf in embryos co-injected with pHuC-zNrf2-HA-2A-mCherry and pHuC-z3R-tau-GFP (b) or pHuC-h4R-tau-GFP (d). For comparison, embryos co-injected with pHuC-mCherry (panels a and c) were used as the control. The boxed regions are enlarged (a′-d′′) to show the GFP-labeled neuronal cells in 24 and 48 hpf embryos from the lateral view. Scale bars: 100 µm. The protection effect of zNrf2 against neuronal death induced by human tau-GFP or zebrafish tau-GFP was presented in panel e to show higher percentage, 58 % and 55 % of zebrafish embryos expressing zNrf2 with more neuronal cells, compared to 19 % and 17 % without zNrf2. b GFP signals (panel a) and mCherry signals (panel a′) in neuronal cells and axons in embryos co-injected with pHuC-h4R-tau-GFP and pHuC-zNrf2-HA-2A-mCherry were colocalized (panel a′′). Scale bar: 50 µm. c Double immunostaining of h4R-tau-GFP (GFP antibody) and zNrf2-HA (HA antibody, panel a′) in spinal cord neurons of the aforementioned zebrafish embryos. The phosphorylation state of h4R-tau-GFP was detected using antibody pT212 (panel b) and antibody AT8 (panel b′). Scale bar: 50 µm |
|
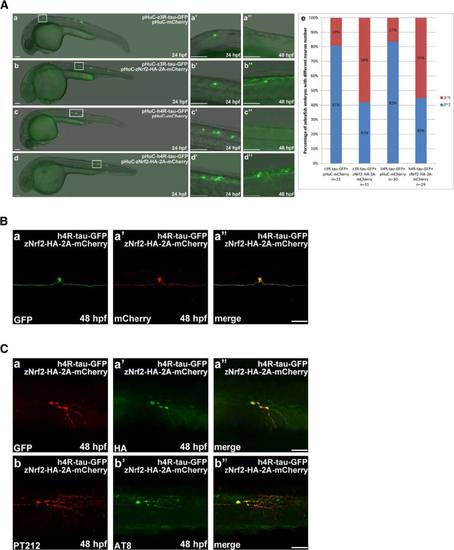
Zebrafish GDNF overexpression prevented human 4R-tau-GFP and zebrafish 3R-tau-GFP induced Neuronal death. a GFP-labeled neuronal cells and axons were observed at 24 and 48 hpf in embryos co-injected with pGFAP-zGDNF-HA and pHuC-z3R-tau-GFP (b) or pHuC-h4R-tau-GFP (d). For comparison, embryos co-injected with pGFAP-zBDNF-HA (panels a and c) were used as the control. The boxed regions are enlarged (a′-d′′) to show the GFP-labeled neuronal cells in 24 to 48 hpf embryos from the lateral view. Scale bars: 100 µm. The protection effect of GDNF against neuronal death induced by human tau-GFP or zebrafish tau-GFP was presented in panel e to show higher percentage, 55 % and 53 % of zebrafish embryos expressing GDNF with more neuronal cells, compared to 20 % and 19 % without GDNF. b Double immunostaining of h4R-tau-GFP (GFP antibody, panel a) and GDNF-HA (HA antibody, panel a′) in spinal cord neurons of the aforementioned zebrafish embryos. The phosphorylation state of h4R-tau-GFP was detected using antibody pT212 (panel b) and antibody AT8 (panel b′). Scale bar: 50 µm |
|
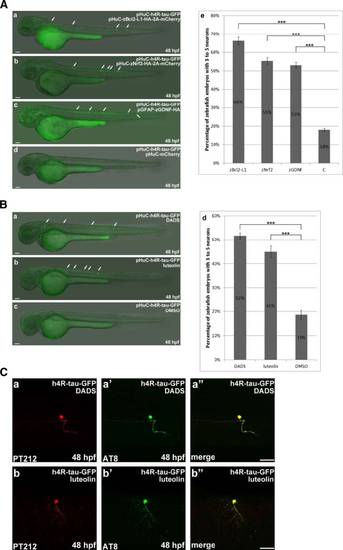
DADS and luteolin treatment prevent neuronal death induced by overexpression of h4R-tau-GFP. a In pHuC-h4R-Tau-GFP-injected embryos, which respectively co-expressed Bcl2-L1 (panel a), Nrf2 (panel b), or GDNF (panel c), there are more neuronal cells survived. Statistical analysis (panel e) represents the quantitative results of zebrafish embryos respectively co-expressing Bcl2-L1 or Nrf2 or GDNF to have higher percentage of more neuronal cells compared to the control. The n value is indicated. b Numbers of zebrafish embryos with more neuronal cells were counted as described above for pHuC-h4R-Tau-GFP-injected embryos treated with DADS (diallyl-disulfide) (panel a) and luteolin (panel b). Statistical analysis (panel e) was presented similarly as described above to show that pHuC-h4R-Tau-GFP-injected embryos treated with DADS or luteolin have higher percentage of more neuronal cells compared to the control. c The effects of DADS and luteolin treatment on h4R-tau-GFP-induced neuronal death were confirmed by double immunostaining of GFP-labeled neurons at 48 hpf. The phosphorylation state of h4R-tau-GFP was detected by antibody pT212 (panels a and b) and antibody AT8 (panels a′ and b′). Embryos are shown from the lateral view with the anterior to the left and dorsal to the top. Scale bar: 50 µm |