- Title
-
Genetic evidence for shared mechanisms of epimorphic regeneration in zebrafish
- Authors
- Qin, Z., Barthel, L.K., and Raymond, P.A.
- Source
- Full text @ Proc. Natl. Acad. Sci. USA
|
Cone photoreceptor regeneration in adult zebrafish. Flat-mounted zebrafish retinas are immunolabeled with cone-specific zpr-1 (red). Retinas are oriented dorsal up, ventral down, nasal left, and temporal right. (A, B) Intact retina. Asterisk, attached retinal pigment epithelium. (D, E) At 3 days after exposure to intense light, cones are missing in a horizontal band across the retina. (G, H) By 14 days, cones have regenerated within the lesioned region (dashed lines). (C, F, and I) Magnified images of the boxes in B, E, and H, respectively. (Scale bars: 300 μm in A, B, D, E, G, and H; 20 μm in C, F, and I.) |
|
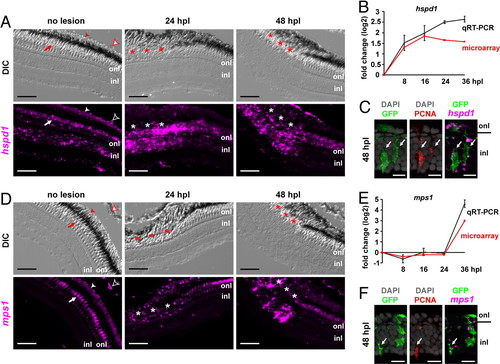
hspd1 and mps1 are up-regulated in injury-activated Müller glia during zebrafish photoreceptor regeneration. Expression patterns of hspd1 (A–C) and mps1 (D–F). (A and D) Fluorescent in situ hybridization of hspd1 and mps1 on retinal sections of Tg(gfap:GFP)mi2002 zebrafish. Autofluorescence in cones (arrow), rods (solid arrowhead), and red blood cells (empty arrowhead). Asterisks, lesioned area (note the disrupted retinal pigment epithelium). DIC, differential interference contrast. (B and E) Expression fold changes of hspd1 and mps1 in isolated GFP+ cells detected by qRT-PCR (gray) and microarray (red). Error bars represent SEM for 3 independent biological replicates. (C and F) Within the lesioned region at 48 hpl: in situ hybridization with hspd1 and mps1, respectively (magenta), produces discrete fluorescent dots associated with GFP+ neurogenic Müller glia (green) and anti-PCNA (red). Arrows indicate triple-labeled cells; onl, outer nuclear layer; inl, inner nuclear layer. These are not microglia, which are confined to the onl in the lesioned region (23). (Scale bars: 50 μm in A and D; 10 μm in C and F.) |
|
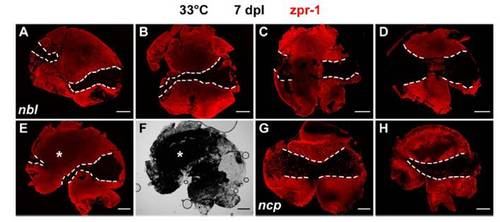
Cones fail to regenerate in nbl and ncp mutants at the restrictive temperature. Flat-mounted retinas at 7 dpl immunolabeled with zpr-1 (red). (A and C) Regenerated cones between dashed lines in WT. (B and D) Few or no cones are seen in the lesioned central area in nbl and ncp, respectively. The occasional zpr-1+ profile in the region of the lesion might represent a spared cone photoreceptor. (Scale bars: 300 μm.) |
|
Retinal regeneration defects of nbl and ncp. (A) Neurogenic clusters at 2 dpl in the inner nuclear layer (inl) immunolabeled with anti-PCNA (magenta) and weakly labeled with anti-Pax6 (green) in WT and nbl. Note that Pax6 is also expressed at high levels in amacrine cells at the inner boundary of the inl. (B) PNCA+ photoreceptor progenitors at 3 dpl in the outer nuclear layer (onl) of WT and ncp. (C) Number of PNCA+ cells in the inl or onl per 100 μm of linear length retina at 2 or 3 dpl, respectively. Error bars represent SEM for 3 individuals. *, P < 0.05; **, P < 0.0001. (D) Transmission electron micrographs of injury-activated Müller glia in WT and nbl. See text for description of temperature shift paradigm. Müller glia (M) are shown by the magenta wash. Mitochondria (arrows) in Müller glia of WT appear normal after 8 h at 33°C, whereas in nbl mutants, Müller glia contain swollen mitochondria. (Scale bars: 10 μm in A and B; 100 μm in D.) |
|
Isolation of GFP+ Müller glia. (A) Dissociated GFP+ Müller glial cell (green). Counterstained with DAPI (blue). (B and C) Flow cytometry scatter plots. forward scatter-height (FSC-H); side scatter-height (SSC-H). Dissociated cells from adult Tg(gfap:GFP)mi2002 zebrafish retinas were gated by forward and side scatters (B), and GFP+ Müller glia were isolated based on fluorescence in the FITC channel (R5) (C). Our yield of dissociated retinal cells from adult zebrafish (5- to 6-month old) was ~2.5 x 105 cells/retina, of which ~9% were GFP+ Müller glia. With flow cytometry, we could recover ~2.1 x 104 Müller glia/retina, representing an efficiency of ~84%. (Scale bar: 10 μm.) |
|
Cone regeneration defect in nbl and ncp mutants at the restrictive temperature. (A–E, G, and H) Flat-mounted retinas at 7 dpl immunolabeled with zpr-1 (red). (A–E) One retina from each of 5 nbl mutants. (F) Bright-field image of E. (G and H) One retina from each of 2 ncp mutants. Dashed lines, light-damaged areas that have few or no zpr-1–labeled cones; we cannot determine from these preparations whether the rare scattered cones sometimes observed within the light-damaged areas survived the lesion or have regenerated. Asterisk, attached retinal pigment epithelium. (Scale bars: 300 μm.) |
|
Transmission electron micrographs of mitochondria in injury-activated Müller glia in WT siblings and nbl mutants after acute exposure to 33°C. (A–C) High-magnification images of mitochondria in injury-activated Müller glia in retinas at 2 dpl after 8 h of exposure to 33°C. See Fig. 4D for lower magnification images of these sections. (A) Glycogen granules (g) and mitochondria (arrows) in Müller glia in WT. (B and C) Swollen mitochondria with empty matrix in Müller glia of nbl. (D) Low-magnification view of a neurogenic cluster (within the arrows) in the inner nuclear layer of nbl at 3 dpl after4hof exposure to 33°C. Asterisks, Müller glia; p, progenitor. Note that the mitochondrial defect is present only in injury-activated Müller glia but not in the associated neuronal progenitors. (E and F) High-magnification images of mitochondria from the Müller glial cells in D. (Scale bars: 0.5 μm in A–C, E, and F; 10 μm in D.) |