- Title
-
Using Light-Sheet Microscopy to Study Spontaneous Activity in the Developing Lateral-Line System
- Authors
- Zhang, Q., Kindt, K.S.
- Source
- Full text @ Front Cell Dev Biol
|
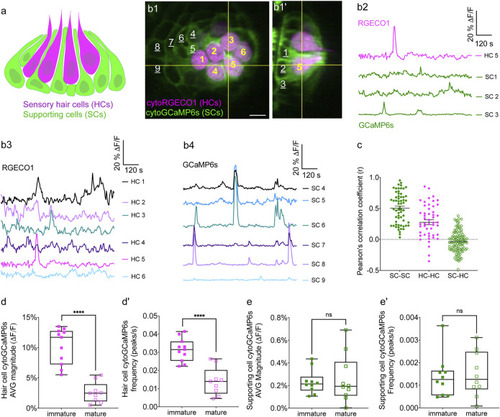
Simultaneous imaging of spontaneous activities in hair cells and supporting cells. |
|
P2yr1 signaling is required for spontaneous activity in supporting cells but not immature hair cells. The spatial patterns of the mean spontaneous calcium activities of the supporting cells |
|
Two-color imaging of spontaneous activities in hair cells and cholinergic efferent terminals. |
|
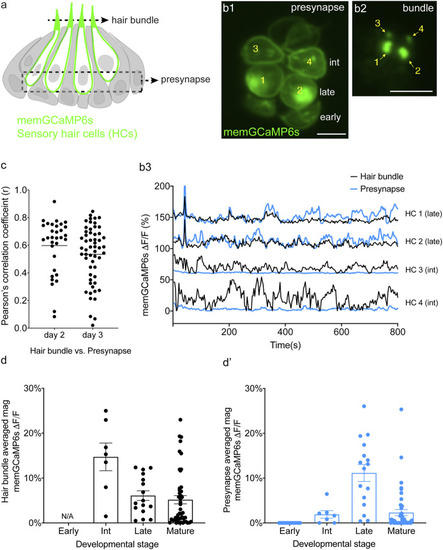
Spontaneous calcium activity occurs in the mechanosensory bundle and at the presynapse. |
|
Spontaneous calcium activity at the presynapse requires CaV1.3 channels. |
|
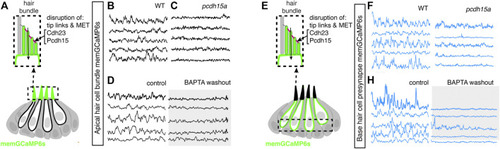
Mechanotransduction in hair bundles is required for spontaneous activity at the presynapse. |
|
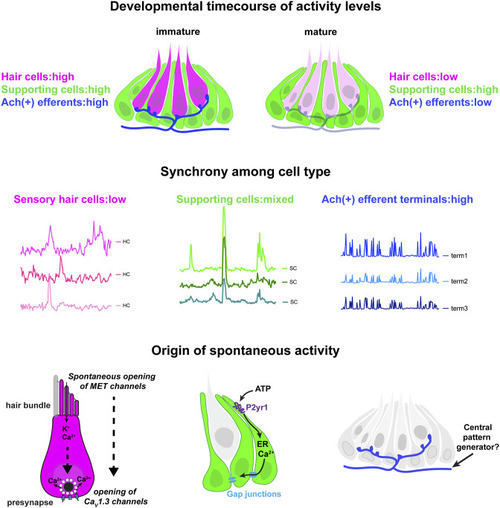
Locations, mechanisms and developmental timecourse of spontaneous calcium activities in the lateral-line system. Top panel: we reliably detected spontaneous calcium activity in three cell types within the lateral-line system: hair cells, supporting cells and cholinergic (Ach (+)) efferent neurons. Spontaneous calcium activity in the supporting cells and cholinergic efferents is not correlated with calcium signals in hair cells. During development spontaneous calcium signals are robust in all three cell types (left side). Upon maturation, spontaneous calcium signals are decreased in hair cells and efferent neurons, but maintained in supporting cells (right side). Middle panel: Within a neuromast spontaneous calcium signals among populations of hair cells are not correlated (left side); supporting cells are moderately correlated (middle); cholinergic terminals are highly correlated (right side). Bottom panel: In developing hair cells, spontaneous opening of MET channels leads to calcium signals in mechanosensory hair bundles. This apical activity triggers opening of CaV1.3 channels at the presynapse, resulting in spontaneous presynaptic calcium signals (left side). In supporting cells, extracellular ATP acts on P2yr1 receptors. P2yr1 signaling leads to calcium release from the ER, giving rise to a spontaneous calcium signal. Spontaneous calcium signals can be propagated to neighboring supporting cells via gap junction channels (middle). Spontaneous activity in cholinergic efferents may be coupled to activity in spinal motor neurons that is present during development in order to form the central pattern generator required for locomotion (right side). |