- Title
-
Zebrafish type I collagen mutants faithfully recapitulate human type I collagenopathies
- Authors
- Gistelinck, C., Kwon, R.Y., Malfait, F., Symoens, S., Harris, M.P., Henke, K., Hawkins, M.B., Fisher, S., Sips, P., Guillemyn, B., Bek, J.W., Vermassen, P., De Saffel, H., Witten, P.E., Weis, M., De Paepe, A., Eyre, D.R., Willaert, A., Coucke, P.J.
- Source
- Full text @ Proc. Natl. Acad. Sci. USA
|
ÁCT images of different zebrafish models with affected type I collagen. For ÁCT scanning, we included several mutants with a nonsense or splice mutation in col1a1a, col1a1b, or col1a2, generating a knockout of these genes (col1a1a?/? not viable). These mutants are grouped together and indicated as ?type I collagen knockout (KO) alleles?quantitative defect? (shown in orange). Another set of mutants carries mutations resulting in substitutions [indicated as ?type I collagen amino acid (AA) substitutions?qualitative defect, shown in blue] of a Gly residue in ?1(I) (col1a1admh13/+, col1a1admh14/+ and col1a1achi/+), ?2(I) (col1a2dmh15/+), or ?3(I) (col1a1bdmh29/+). The microwaved (col1a1amed/med) mutant carries a homozygous Glu substitution in ?1(I). Finally, we included two mutant models with a knockout mutation in the bmp1a and plod2 genes (?KO alleles in genes involved in type I collagen processing,? shown in green). Representative fish from each mutant genotype are shown. Callus formation in ribs (arrowheads), local compressions of the vertebral column (brackets), and kyphosis (arrow), are indicated (also listed in Table 2). PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|
|
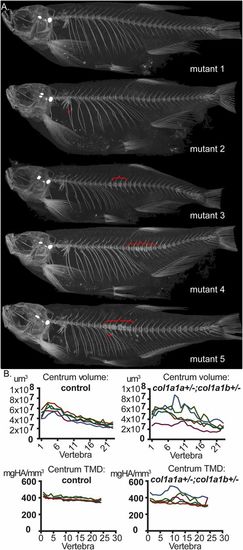
col1a1a+/?;col1a1b+/? mutant fish exhibit intragroup variability in phenotypic expressivity. (A) Different col1a1a+/?;col1a1b+/? mutant siblings were analyzed using ÁCT scanning at 5 mo of age (mutant 1 to mutant 5). Upon comparison of the different mutant siblings, both incomplete penetrance and a variable expressivity of features indicative of compromised bone quality was observed, which include fractures (arrowheads) and local compressions in the vertebral column causing a distorted shape of the vertebral bodies (brackets). (B) Intragroup variability for col1a1a+/?;col1a1b+/? mutant fish is demonstrated for volume and TMD of the vertebral centra, with values in the mutant group showing a much larger spread than compared with the control group. The different colors in one graph represent different individual fish of the same group (mutant siblings or control siblings). PHENOTYPE:
|
|
In-depth analysis of mutants with partial or complete loss of col1a1a and/or col1a1b. (A) Segregation analysis of the different mutant genotypes generated from an in-cross of col1a1a+/?;col1a1b+/? double-heterozygous mutant fish. Progeny were genotyped at three different time points (100 fish per time point): 7 dpf, 15 dpf, and adult stage (3 mo). The relative abundance of each genotype is given for each time point as a percentage. ?Expected? indicates the theoretical abundance of each genotype, following Mendelian segregation. All combinations with a complete loss of col1a1a (col1a1a?/?, indicated in red shades), are less viable and completely lost when progeny reached adulthood. Genotypes that contain a complete loss of col1a1b (col1a1b?/?, indicated in green shades) are still present at adult stage. Black indicates the parental genotype, white indicates wild-type fish, and gray represents the other remaining genotypes. (B) Phenotype of col1a1a?/? mutant larvae at 7 dpf, compared with wild-type siblings. Col1a1a?/? mutant larvae present with a noninflated swimbladder (asterisk) and a ruffled finfold (arrowhead). (C) Ventral view and detail of the pectoral fins showing frilly distal margins (arrowhead) in the pectoral fins of col1a1a?/? mutant larvae at 7 dpf. (D) Detail of the col1a1a?/? mutant larvae finfold at 7 dpf, showing an absence of fin actinotrichia fibers, which can be clearly observed in the finfold of wild-type siblings (arrow). PHENOTYPE:
|
|
In-depth analysis of col1a2+/? and col1a2?/? mutant fish. (A) Visual phenotype of col1a2 mutant and wild-type siblings. Both col1a2+/+ control, and col1a2+/? mutant fish display a normal morphology, while col1a2?/? mutant are dysmorphic and display kyphosis (arrowhead). The typical stripe pattern in the skin is disturbed in col1a2?/? mutant fish, but normal in col1a2+/+ controls and col1a2+/? mutant fish. (B) Masson?s Trichrome staining of histological sections of the skin of an adult col1a2?/? mutant fish and a col1a2+/+ control sibling. Note the much thinner dermis (asterisk), composed of layers of collagen fibrils (stained blue) in col1a2?/? mutant fish compared with col1a2+/+ controls. (C) Average maximum tensile strength, measured by biomechanical testing of skin flaps dissected from five adult col1a2?/? mutant fish and five col1a2+/+ control fish, illustrating strongly decreased strength of soft connective tissues in mutant fish (P < 0.0001). (D) Histological analysis of the vertebral column of adult fish, sagittal sections stained with H&E. Shown at the Left are two adjoining vertebral body endplates (Vep, i.e., the region where two adjacent vertebral bodies meet and are connected by the IVL) in a col1a2+/+ control and a col1a2?/? mutant adult fish. The black rectangles indicate the region which is shown at a higher magnification in the image next to it on the Right, depicting the IVL in detail. In control fish, the IVL is typically composed of three distinct layers or tissues: the notochord sheet (Ns, mainly type II collagen), the outer elastin layer (OEL), and a layer of type I collagen fiber bundles (cI). In the col1a2?/? mutants, IVL composition is abnormal: the OEL was still present, the Ns layer could be observed but was extremely reduced, but no layer of type I collagen fibers (cI) could be observed. Col1a2?/? mutant bone (B) in the vertebral bodies near the Vep showed a loss of typical Sharpey fibers (Sf), while the notochord cells (Nc) displayed a loss of vacuoles, in addition to sclerosis (Sc). As shown in the two rightmost pictures, we observed fusions of adjacent vertebral bodies (black arrowhead) and dislocation of the IVL and Vep (red arrow) in some of the vertebrae, likely resulting in kyphosis seen in col1a2?/? mutant fish. Other abbreviations: Ep, epidermis; Mu, muscle fibers; Ob, osteoblasts; Scl, Scale. |

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|