- Title
-
Grhl1 deficiency affects inner ear development in zebrafish
- Authors
- Liu, F., Yang, F., Wen, D., Xia, W., Hao, L., Hu, J., Zong, J., Shen, X., Ma, J., Jiang, N., Sun, S., Zhang, J., Wang, H., Wang, X., Ma, Z., Ma, D.
- Source
- Full text @ Int. J. Dev. Biol.
|
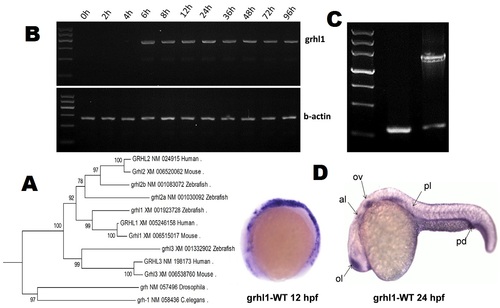
grhl1 mRNA expression in embryonic zebrafish. (A) Phylogenetic analysis indicates that GRHL1 is evolutionarily nearer to GRHL2 than to GRHL3. (B) Grhl1 expression persists from 6 hpf onward in zebrafish. (C) Reverse-transcription PCR indicates considerable knockdown efficiency in the MO group. Primers were designed to work in intron 3 and exon 3. (D) WISH indicates a ubiquity of grhl1 in embryos at 12 and 24 hpf. The signal was detected in the ear region, al, anterior lateral line primordium; ll, lateral line neuromast; ol, olfactory placode; ov, otic vesicle; pd, pronephric duct; pf, pectoral fin; pl, posterior lateral line primordium. |
|
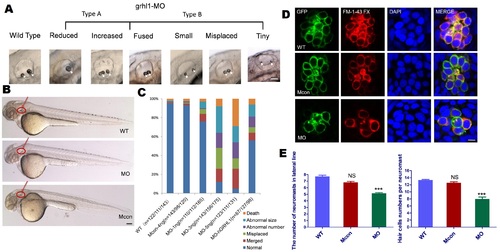
Knockdown of grhl1 induces otolith and neuromast defects in larval zebrafish. (A) Different types of malformed otoliths in the MO group. (B) No significant exterior differences among wide-type embryo and zebrafish injected with MO or mismatch control MO. (C) Statistical results of different types of malformed otoliths over all MO concentrations (3 ng was the optimal concentration). (D–F) Lack of grhl1 led to decreased HCs/neuromasts. Bar: 200 µm in (A,B); 10 µm in (D). PHENOTYPE:
|
|
Lack of grhl1 damages desmosomes and causes hair cell apoptosis (A) Apoptotic signals were observed in hair cells in the MO group. (B,C) Lack of grhl1 damages desmosomes (arrows). (D,E) GRHL1 and DSG1 mRNA could rescue the decreased number of hair cells and malformed desmosomes caused by MO. Bar: 15 µm in (A); 1 µm in (C,D). PHENOTYPE:
|
|
Hearing disability in Hearing disability in grhl1 knockdown zebrafish which could be rescued by DSG1 mRNA. (A) Poorly organized desmosomes (arrows) in the MO group could be rescued by DSG1 mRNA. (B) Dsg1 rescued decreasing numbers of neuromasts in the lateral line. (C) Examples of C-shaped startle responses of different larval groups in response to a 500 Hz sound of 10 ms duration. Typical escape response of wild-type larva initiated 10 ms after stimulation. (D) Average C-startle response probability after sound stimulation. At least 40 larvae were tested for each group. Bar, 1 mm. knockdown zebrafish which could be rescued by DSG1 mRNA. (A) Poorly organized desmosomes (arrows) in the MO group could be rescued by DSG1 mRNA. (B) Dsg1 rescued decreasing numbers of neuromasts in the lateral line. (C) Examples of C-shaped startle responses of different larval groups in response to a 500 Hz sound of 10 ms duration. Typical escape response of wild-type larva initiated 10 ms after stimulation. (D) Average C-startle response probability after sound stimulation. At least 40 larvae were tested for each group. Bar, 1 µm. PHENOTYPE:
|
|
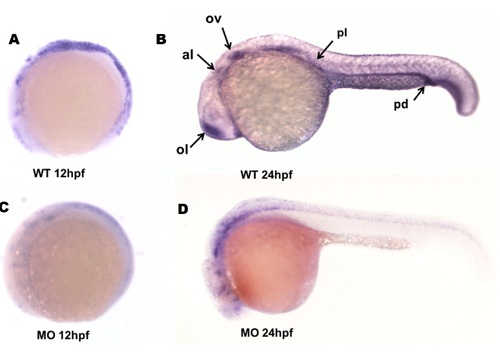
WISH was performed to verify the efficiency of MO. |
|
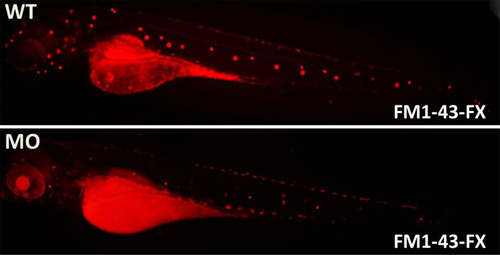
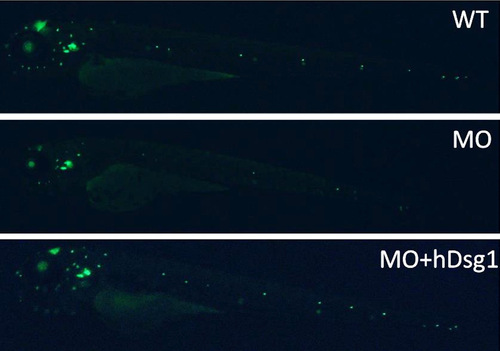
FM-143-FX staining indicates that neuromasts are not as bright as in the WT group. |
|
Neuromasts, originating from a primordium near the head, are neatly arranged from L1 to L8 (WT). |
|
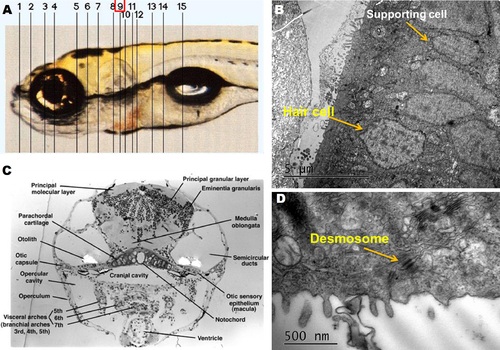
The systematical sketch for identifying sensory epithelium. (A) Lateral view of the 120 hour embryo. (B,C) Serial section was performed and the 9th layer of the lateral view could find the supporting cell and hair cell. (D) A desmosome was found by TEM in the 9th layer. |