- Title
-
Bioelectric signaling regulates size in zebrafish fins
- Authors
- Perathoner, S., Daane, J.M., Henrion, U., Seebohm, G., Higdon, C.W., Johnson, S.L., Nüsslein-Volhard, C., and Harris, M.P.
- Source
- Full text @ PLoS Genet.
|
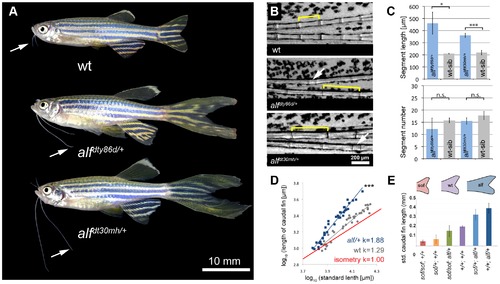
alf mutants lead to an increase in size of the appendages of adult fish. (A) alf mutations are dominant and lead to overgrown fins and barbels in the adult. Arrows indicate maxillary barbels; the mutants shown are heterozygous. (B) Segment patterning in the dorsal fin of wild type and heterozygous mutants. Brackets indicate one segment. Although the majority of segments show increased length, several short segments can be seen in the mutants (arrows). (C) Variation in segment length (top) and segment number (bottom) in the longest ray of the dorsal fin of mutants and wild type siblings (wt sib). Fish of similar standard length (SL) (i.e. distance between snout and caudal peduncle) were compared; all cases n = 4; error bars: standard deviation; n.s.: not significant; *: p<0.02, ***: p<0.001. (D) Increased allometric scaling of heterozygous alf fins in development. k = allometric coefficient, Linear regression lines, wt R2 = 0.92; alf/+, R2 = 0.95; ***: p<0.001. (E) Crosses of sof with alf indicate that there is not epistatic interaction between the two genes. Fin length was normalized with SL. PHENOTYPE:
|
|
Cell proliferation is increased in alf mutants. (A) Sections of wild type and heterozygous alf fins. No significant difference in cell size is seen in the two groups. (B) Antibody staining against PCNA on paraffin sections of regenerating fins 4 days post amputation (dpa). Chart shows percentage of proliferating nuclei (PCNA) over total nuclei (Hoechst). N = 3?4 sections of 4 individual fish **: p-value<0.01. PHENOTYPE:
|
|
The alf phenotype is due to gain-of-function mutations within the K+ channel kcnk5b. (A) alf mutations map to chromosome 20 between z11841 and z21067. Gray: north markers; blue: south markers. (B) Electropherogram of kcnk5b at position 169 and 241 in mutants and wild type siblings. (C) The amino acids affected in the mutants are well conserved among vertebrates. (D) A revertant of alfdty86d (j131x8) shows wild type-sized fins. (E) kcnk5bj131x8 fish harbor an intragenic deletion in kcnk5b that is predicted to cause a truncated protein lacking three transmembrane (TM) domains. PHENOTYPE:
|
|
Vertebrate kcnk5 homologs and expression in zebrafish development. (A) Due to a whole genome duplication event, teleost fish have two kcnk5 paralogs that show early divergence. Numbers indicate bootstrap values in percentage (100 bootstrap replications). Nodes with a bootstrap value lower than 95 were collapsed. Dre, Danio rerio; Ola, Oryzias latipes; Gac, Gasterosteus aculeatus, Tru, Takifugu rubripes; Tni Tetraodon nigridoviridis, Gmo, Gadus morhua; Mmu, mus musculus; Gga, Gallus gallus; Xtr Xenopus tropicalis. (B) RT-PCR of kcnk5a and kcnk5b shows comparable expression between the two paralogs in multiple adult tissues, including fins. |
|
Overexpression of kcnk5b is sufficient to cause fin overgrowth. (A) Construct used to create kcnk5b-expressing clones via Tol2 transgenesis. (B) Individual fish expressing kcnk5b (W169L) (left) or kcnk5b (wt) (right) in mosaic clones display localized fin and barbel overgrowth. (C?F) Overgrowth is associated with DsRed expression (in red) within mesenchymal cells. (C) Calcein staining labels bone tissue (in green) of an overgrown fin (DsRed; kcnk5(W169L) expressing clone). (D) Mesenchymal clones are associated with increased segment length in the fin compared to non-overgrown DsRed negative regions. (E) Fibroblast-like cells appear as DsRed positive cells within the fin rays (dotted line) that surround DsRed negative vasculature (arrows in E and F) which extend along the actinotrichia (fibrils within dotted lines in F) towards the distal end of the fin. (G) Overgrown barbels show DsRed signal within the mesenchyme (area within dotted line) but not in the vasculature (arrow). (H) Number of clones associated with overgrowth in different kcnk5b variants. (I) Proportion of different cell types labeled in overgrown tissues. (J) Electrophysiological recordings of the non-conductive kcnk5b (GFGAAA) mutant in oocytes. Squares: kcnk5b (wt), purple stars: kcnk5b (F241Y)+kcnk5b (wt), blue circles: kcnk5b (W169L)+kcnk5b (wt), green triangles: + kcnk5b (GFGAAA)+kcnk5b (wt). Current was normalized to the measurement of wt current at 60 mV. Inset: DsRed+ fibroblasts in fish injected with the non-conductive construct do not lead to fin overgrowth. |
|
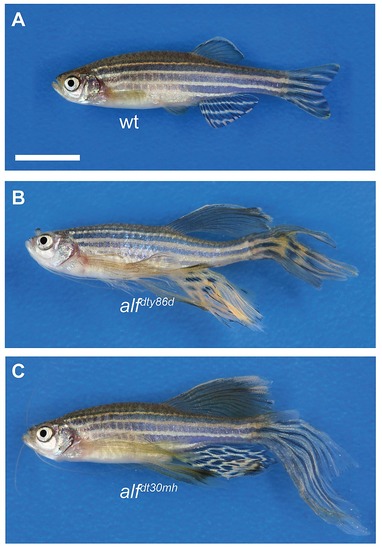
Phenotype of homozygous alf mutants. (A) wt, (B) alfdty86d homozygous, (C) alfdt30mh homozygous. Scale bar: 10 mm |
|
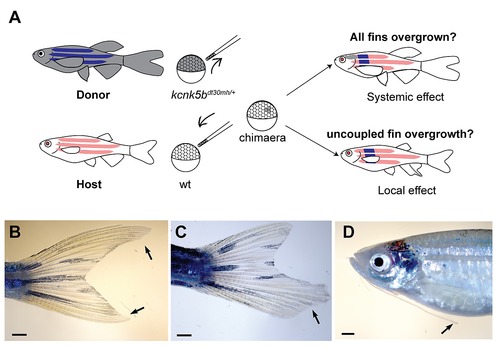
kcnk5b gain-of-function mutations affect local growth of appendages. (A) Transplantation of kcnk5bdt30mh/+ cells into wt albino hosts. If the mutation acts on a systemic level, mutant clones should promote overgrowth of all appendages. If the mutation has a local effect, overgrowth will be observed in patches. Chimeras resulting from the transplantation experiments show overgrowth of (B) single fins, (C) fin parts or (D) individual barbels. |
|
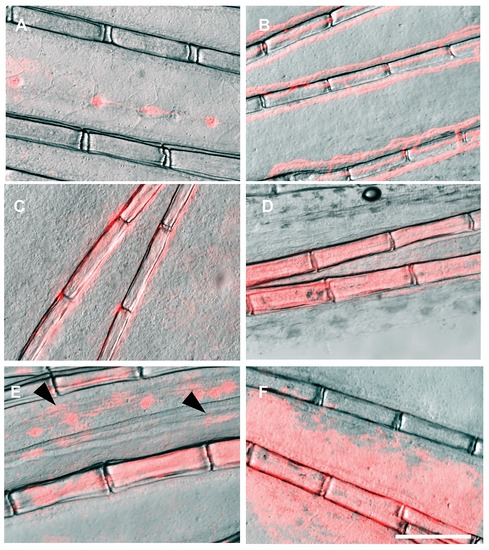
The control plasmid ef1a:DsRed drives DsRed expression in a wide range of cell types and tissues within the fin. (A) lateral line, (B) vasculature, (C) osteoblasts, (D) fibroblasts, (E) pigment cells (arrows), showing the typical stellated shape, and (F) epidermis. Scale bar: 200 μm |
|
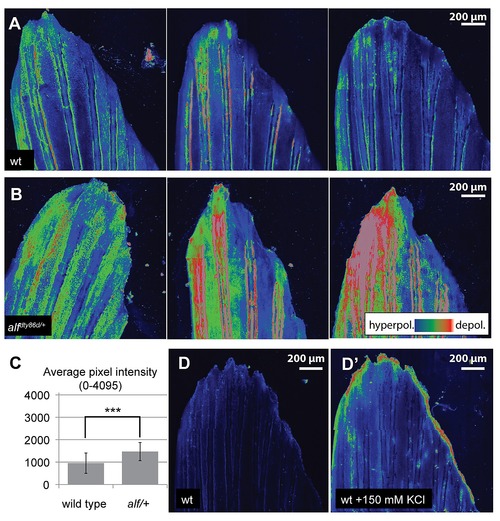
Polarization of fins during growth. Voltage sensitive dyes were used to assess changes in overall polarization of growing caudal fins of wild type and alf juvenile fish. (A) DiSBAC2(3) staining in wild type fins exhibited hyperpolarization localized to discrete regions of the fin with variable detection of distal regions of altered depolarization. (B) alf fins in contrast show high levels of depolarization across the fin with variable patterns in different tissues. (C) Quantification of average DiSBAC2(3) fluorescence signal in wild type and mutant fins (average pixel intensity (12-bits) of the fin in maximum intensity projections). ***: p<0.001, N = 21?23. (D) Positive control of depolarization by treatment of the fins with 150 mM KCl (D2). DiBAC4(3), another dye sensitive to depolarization, showed similar effects, while DiSC3(5), a dye sensitive to hyperpolarized states, was uninformative (data not shown). |