- Title
-
Arterial and venous progenitors of the major axial vessels originate at distinct locations
- Authors
- Kohli, V., Schumacher, J.A., Desai, S.P., Rehn, K., and Sumanas, S.
- Source
- Full text @ Dev. Cell
|
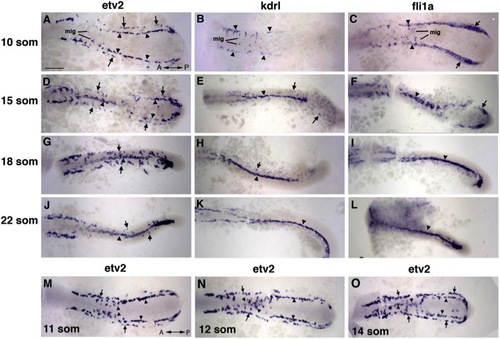
Medial and Lateral Angioblasts Originate in Two Distinct Regions, as Observed by ISH Analysis for etv2 Expression (A?L) etv2, flk1/kdrl, and fli1a expression analysis in trunk angioblasts at the 10-, 15-, 18-, and 22-somite stages. etv2 expression is observed in the medial (arrowheads) and lateral (arrows) angioblasts. flk1/kdrl and fli1a expression is initially restricted to the medial angioblasts (B and C) with very weak observed expression in the lateral angioblasts in 15- to 22-somite stage embryos (E?K; F?L). (M?O) etv2 expression in migrating medial angioblasts at the 11- to 14-somite stages. Note that the migration wave progresses in the anterior to posterior direction. The lateral angioblasts migrate later at the 16- to 22-somite stages (G and J). Dorsal view of the trunk and tail region of flat-mounted embryos. Anterior is to the left. Scale bar, 100 μm. See also Figures S1 and S2, and Movies S1, S2, S3, and S4. |
|
Two-Color ISH Expression Analysis of etv2 and Other Endothelial and Hematopoietic Markers ISH expression of (A and B) etv2 and gata1 at the 15-somite stage, (C) etv2 and kdrl at the 16-somite stage, (D) etv2 and scl at the 10-somite stage, and (E and F) etv2 and grl at the 16-somite stage. (A and B) Erythroid-specific gata1 expression (white arrows) is positioned between the medial (arrowheads) and lateral (black arrows) etv2-expressing angioblasts; (B) higher magnification view. (C) etv2 and kdrl expression overlaps at the midline (arrowhead) in the medial angioblasts. While strong etv2 expression is seen in the lateral angioblasts (arrow), no or very little lateral kdrl expression is observed. (D) Relative to vascular endothelial progenitor cells, erythroid-specific scl expression (white arrows) is positioned between the medial (arrowheads) and lateral (black arrows) etv2-expressing angioblasts. (E and F) Similar to (C), grl expression overlaps with etv2 expression in medial angioblasts at the midline (arrowheads). Lateral angioblasts are grl-negative (arrows). Note that the strong bilateral grl expression (*) does not correspond to endothelial cells and is within the somitic tissue as reported previously (Zhong et al., 2000). See also Movies S1, S2, S3, and S4. |
|
The Migration of Trunk Angioblasts in Live etv2:GFP-Transgenic Embryos(A?G) GFP expression is observed in the medial and lateral endothelial progenitor cells at the 10- to 16-somite stages. In laterally (A) and dorsally mounted embryos (B?G), medial angioblasts (white arrows) migrate toward the midline (white arrowheads) intersomitically. (B?G) Selected frames from a time-lapse movie showing migration of the medial angioblasts; imaging was started at the 10-somite stage. The anterior angioblasts migrate first, followed by the more posterior endothelial progenitor cells. (F and G) Faint GFP expression in the lateral angioblasts (red arrowheads) becomes apparent; these cells migrate to the midline similar to the medial angioblasts. In (A) anterior is to the left; in (B)?(G) anterior is up. Scale bar, 100 μm. See also Figure S2 and Movies S1, S2, S3, and S4. |
|
The Medial Angioblasts Give Rise to the Dorsal Aorta, while the Lateral Angioblasts Give Rise to the Posterior Cardinal VeinIn all experiments, etv2:Kaede embryos were photoconverted at the 14- to 15-somite stages and analyzed at 24?28 hpf. Anterior is to the left; dorsal is up in all panels. (A and B) Global green to red photoconversion of etv2:Kaede-positive cells demonstrates that all early Kaede+ endothelial progenitor cells that were photoconverted contribute to the DA (observed as an overlap of red and green fluorescence), while all late Kaede+ cells that originated after photoconversion contribute to the PCV (observed as green-only fluorescence). Note that etv2:Kaede expression pattern is mosaic, and not all endothelial cells express Kaede. In addition, etv2:Kaede is expressed in certain hematopoietic lineages such as neutrophils (red round cells in A and B), which were not included in the analysis.(C and D) Selected groups of medial etv2:Kaede cells were photoconverted using laser confocal microscopy at the 15-somite stage (arrow, C), and their location was analyzed at 26?28 hpf (arrows, D) using fluorescence microscopy. Note that the labeled medial cells exclusively contribute to the DA, including DA-derived intersegmental vessels (ISV, D). Arrowheads in (C) point to the cells of the lateral line. Red round cells apparent on the right side of (D) correspond to etv2:Kaede expression in hematopoietic lineages. Panels in (C) and (D) represent different embryos. (E) Global photoconversion of etv2:Kaede in VegfA-RNA-overexpressing embryos. Note that the cells in the DA and PCV positions include both double-positive (red and green) medial-line-derived endothelial cells and green-only lateral-line-derived endothelial cells (arrowheads). (F) VegfA MO-injected embryos display a single vessel, as observed by global etv2:Kaede photoconversion. Double-positive (red and green) medial-line-derived endothelial cells are largely located in the dorsal most part of the vessel, while green-only lateral-line-derived cells are found largely in the ventral part of the vessel. (G) Shh RNA-injected etv2:Kaede globally photoconverted embryos display a significant number of green-only lateral-line-derived cells in the DA (arrowhead) and occasional medial-line-derived cells in the PCV (arrow, points to a small double-positive endothelial cell). (H) Cyclopamine (CyA)-treated etv2:Kaede globally photoconverted embryos have disorganized cells within the vascular cord often with no discernable distinction between the DA and the PCV. Higher frequency of green-only cells can be observed in the dorsal part of the vessel (arrowheads). (I) Average contribution of medial (red) and lateral (green) globally photoconverted etv2:Kaede cells to the DA and the PCV in wild-type (n = 13 embryos and 335 endothelial cells analyzed), VegfA RNA (n = 17 embryos and 417 cells), Vegf MO (n = 10 embryos and 295 cells), Shh RNA-injected (n = 17 embryos and 247 cells), and CyA-treated (n = 8 embryos and 88 cells) embryos. Cell counts for each embryo are approximate estimates. Embryos treated with 1% DMSO (solvent for CyA) displayed distribution of green and red Kaede+ cells, similar to WT (not shown). In addition to the flat elongated vascular endothelial cells, etv2:Kaede expression was observed in round nonendothelial cells, which most likely correspond to hematopoietic progenitors such as neutrophils (data not shown); these cells were excluded from the analysis. *Statistically significant differences, as calculated using Z-test for two proportions to compare the percentage of green cells contributing to the DA or the percentage of red cells contributing to the PCV between the treated embryos and wild-type controls (p d 0.001). See also Figure S3. |
|
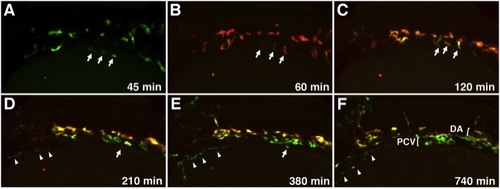
Migration of Medial and Lateral Angioblasts to the DA and PCV Observed in Live etv2:Kaede Embryos Selected frames from a time-lapse movie of a single etv2:Kaede embryo prephotoconversion at the 14-somite stage (A) and postphotoconversion starting at the 15-somite stage (B) through 28 hpf (F). Arrows mark laterally positioned angioblasts that migrate directly to a ventral position to contribute to the PCV. Arrowheads mark a separate population of lateral angioblasts that contribute directly to the common cardinal vein (CCV). All views are dorsolateral; anterior is to the left, and dorsal is to the top. All selected frames are from Movie 6, embryo 1; imaging (t = 0 min) was started at approximately 13-somite stage. See also Movies S5, S6, S7, and S8. |
|
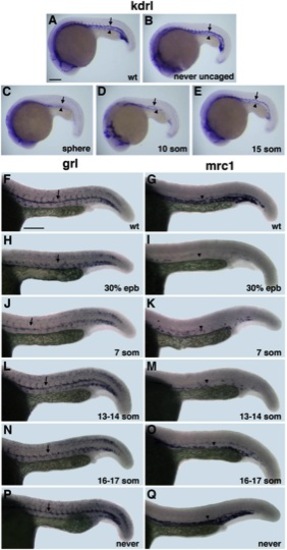
Arterial-Venous Differentiation Is Affected by Etv2 Function at Distinct Time Points (A?E) flk1/kdrl expression analysis at 22 hpf stage. Control uninjected (A), MO-injected but never uncaged (B), uncaged at the sphere (C), 10-somite (D), and 15-somite (E) stage embryos. Note that both the dorsal aorta (arrows) and the posterior cardinal vein (arrowheads) are absent in (C); the dorsal aorta is partially (D) or fully present (E), while the vein is greatly reduced or absent. (F?Q) Arterial grl (F, H, J, L, N, and P) and venous mrc1 (G, I, K, M, O, and Q) expression at 24 hpf in photoactivatable etv2 MO-injected embryos. (F and G) Control uninjected, (H and I) uncaged at 30% epiboly, (J and K) 7-somite stage, (L and M) 13 to 14-somite stage, (N and O) 16- to 17-somite stage, and (P and Q) MO-injected but never uncaged embryos. Note that arterial grl expression (arrows) is absent in (H) but is partially present in (J) and not significantly affected when MO is photoactivated at the 13- to 17-somite stages (L and N). Venous mrc1 expression (arrowheads) is absent or strongly reduced in both early (I and K) and late photoactivated embryos (M and O). Scale bar, 100 μm. See also Figure S4. |
|
In situ hybridization analysis for etv2, ephrin B2a, and ephB4. (A,B) Etv2 expression in wild type embryos at 4 somite (A) and 8 somite stages (B). Note that etv2 expression is observed in the medial angioblasts (arrowheads) only. Flat mounted embryos, dorsal view, A ? anterior; P ? posterior. (C-F) Arterial expression of ephrin B2a (C,E) and venous expression of ephb4a (D,F) is absent in etv2y11-/- mutant (E) or morphant (F) embryos at 24-27 hpf. Arrows in (C) indicate expression in the dorsal aorta. Arrowheads in (D) indicate expression in the posterior cardinal vein. |
|
The intersomitic migration of angioblasts in photoactivated Kaede mRNA injected embryos and Tg(fli1a:GFP) embryos. (A-F) Images extracted from time lapse video depict the intersomitic midline migration of photoconverted angioblasts. (A) Brightfield image before photoactivation showing angioblasts (arrowheads) clustering at the boundary of adjacent somites (S). Arrow depicts angioblasts ingressing into the somitic boundary. (B-F) In Kaede mRNA injected embryos, a cluster of cells that included anterior trunk angioblasts were photoconverted for differential labeling. Following photoconversion, the time progression shows activated angioblasts migrating between somites (C-E; arrowhead) towards the midline (F). Some cells in the somitic mesoderm were labeled as well during photoconversion. Scale bar for (A) and (B-F) represent 50 and 100 μm, respectively. A ? anterior; P ? posterior; S ? somites. (G-J) Images extracted from time lapse confocal imaging of a dorsally mounted fli1a:GFP embryo showing angioblast and erythroid cell migration. Red arrows depict the intersomitic migration of angioblasts towards the midline. Insets show magnified views of migrating angioblasts. |
|
Tg(etv2:Kaede) fluorescence in trunk angioblasts at the 15- somite stage. Note that Kaede expression is only apparent in medial angioblasts most of which are at the midline at this stage. Dorsal view, anterior is to the left. |
|
The effects of photoactivatable etv2 MO on Tg(etv2:GFP) fluorescence and etv2 expression. (A-D) Photoactivatable etv2 MO inhibits etv2-2:GFP fluorescence. (A) Control uninjected, (B) regular etv2 MO2 injected embryo, (C) caged etv2 MO injected embryo uncaged at the sphere stage (4 hpf), (D) caged etv2 MO injected embryo never uncaged. All embryos were analyzed at 24 hpf with the same imaging and acquisition settings. (C) Embryos that were uncaged at the sphere stage resulted in nearly complete inhibition of GFP expression, showing a similar phenotype to standard etv2 MO injected embryos. (D) Caged MO injected embryos that were never uncaged showed higher GFP fluorescence than (C) uncaged embryos. While caged MO injected embryos that were never uncaged (D) showed a decrease in GFP expression in comparison to (A) uninjected embryos, these embryos were phenotypically normal with no apparent vascular defects. (E-H) etv2 expression analyzed in flat mounted embryos at the 22 to 24-somite stage in (E) control uninjected, (F) caged MO injected embryos that were uncaged at the sphere, (G) 5-somite and (H) 10-somite stage. Dorsal view, trunk and tail region is shown. (E) Medial (arrowhead) and lateral (arrows) angioblasts are shown migrating, and many cells have reached the midline. (F, G) Midline migration is absent in embryos that were uncaged at the sphere, and the 5-somite stage. (H) In embryos uncaged at the 10-somite stage, the medial angioblasts migrate to the midline while the migration of lateral angioblasts is inhibited. |
Reprinted from Developmental Cell, 25(2), Kohli, V., Schumacher, J.A., Desai, S.P., Rehn, K., and Sumanas, S., Arterial and venous progenitors of the major axial vessels originate at distinct locations, 196-206, Copyright (2013) with permission from Elsevier. Full text @ Dev. Cell