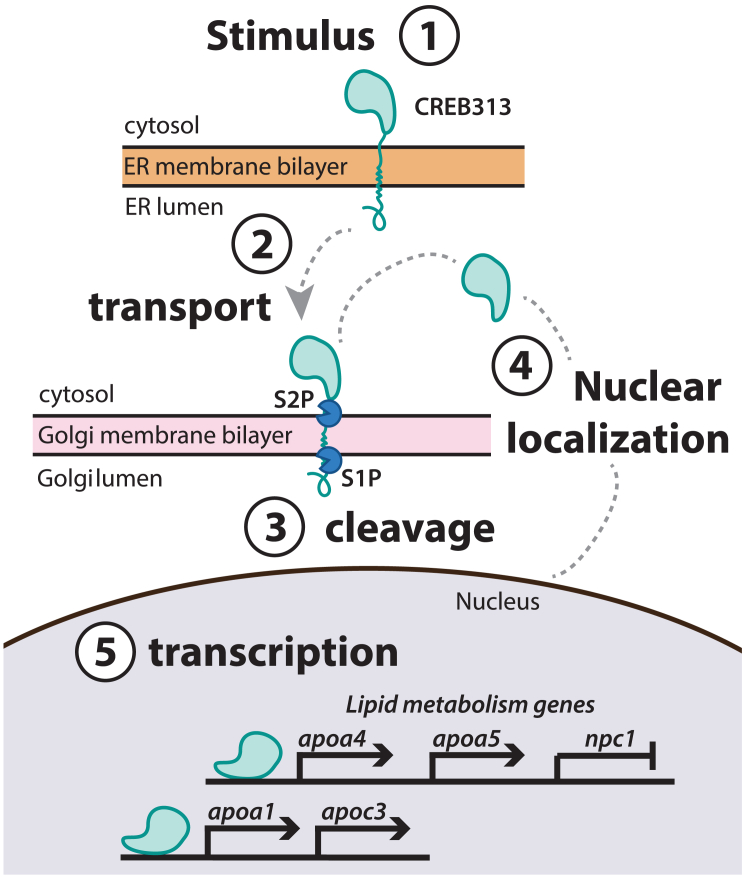
Fig. 1
Process of CREB3L3 nuclear localization. 1: In the inactive state, CREB3L3 is anchored to the membrane of the endoplasmic reticulum (ER). 2: An unknown signal induces the release of the tethered protein from the ER membrane, and CREB3L3 is transported to the Golgi. 3: Proteases (S1P, S2P) cleave the transmembrane domain of the protein in the Golgi. 4: Upon cleavage, the DNA-binding domain of the protein can be trafficked to the nucleus. 5: CREB3L3 regulates expression of key lipid metabolism and apoprotein genes by binding to promoter regions and altering transcriptional processes in the nucleus. CREB3L3, cAMP-responsive element-binding protein 3–like 3.