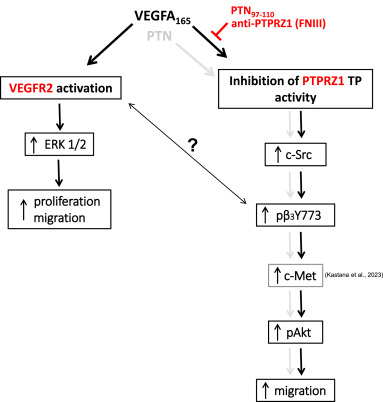
Fig. 9 Schematic representation of the pathway proposed by the present study, by incorporating knowledge from our previous studies, as shown. In brief, VEGFA165 or PTN binds to the FNIII domain of PTPRZ1 and inhibits its tyrosine phosphatase activity, leading to c-Src activation, phosphorylation of β3 integrin at Tyr773, activation of c-Met and Akt, and activation of endothelial cell migration. VEGFA165 activates endothelial cells by also binding to VEGF receptor 2. The exact role of the interaction of β3 integrin with VEGFR2 needs to be further studied. Inhibition of VEGFA165 binding to PTPRZ1 by our identified PTN97-110 peptide or an anti-PTPRZ1 antibody against the PTPRZ1 FNIII domain inhibits the stimulatory effects of VEGFA165 and PTN on cell migration and warrants further exploitation for the development of novel therapeutics.
Reprinted from European Journal of Pharmacology, 977, Choleva, E., Menounou, L., Ntenekou, D., Kastana, P., Tzoupis, Η., Katraki-Pavlou, S., Drakopoulou, M., Spyropoulos, D., Andrikopoulou, A., Kanellopoulou, V., Enake, M.K., Beis, D., Papadimitriou, E., Targeting the interaction of pleiotrophin and VEGFA165 with protein tyrosine phosphatase receptor zeta 1 inhibits endothelial cell activation and angiogenesis, 176692, Copyright (2024) with permission from Elsevier. Full text @ Eur. J. Pharmacol.