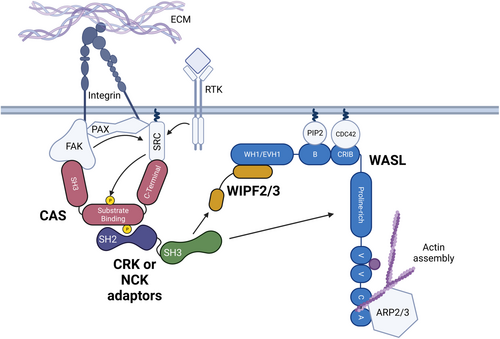
Fig. 8 Model of CRK and NCK family overlapping function in cell motility and adhesion pathways. Activation of integrin receptors by binding to the extracellular matrix (ECM) or autophosphorylation of receptor tyrosine kinases (RTK) through ligand binding leads to the activation of SRC (arrows). Upon integrin receptor activation, Focal Adhesion Kinase (FAK), Paxillin (PAX), SRC, CRK-Associated Substrate of SRC (CAS), and other proteins cluster to form focal adhesions – Centers of adhesion and migratory signaling near the cell membrane. SRC primarily phosphorylates (yellow) CAS on tyrosine – X-X-proline motifs; CAS contains 15 Y-X-X-P motifs in its substrate binding domain. Tyrosine phosphorylation allows CRK or NCK family adaptor proteins to bind via their SH2 domain (purple). The CRK/NCK SH3 domain (green) binds either directly to WASL via its proline-rich domain or indirectly to WIPF2 or WIPF3. WASL's proximity to the focal adhesion drives localized Actin polymerization through the ARP2/3 complex.
Image
Figure Caption
Acknowledgments
This image is the copyrighted work of the attributed author or publisher, and
ZFIN has permission only to display this image to its users.
Additional permissions should be obtained from the applicable author or publisher of the image.
Full text @ FEBS Lett.