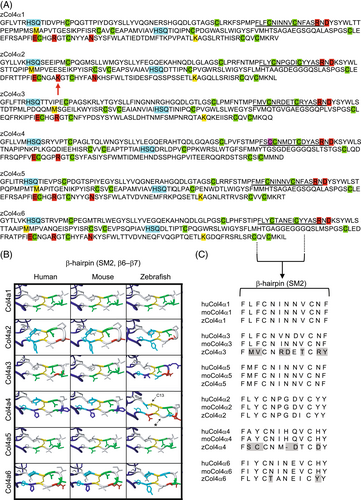
Fig. 3 Structure of the NC1 domain of zebrafish collagen IV α-chain proteins. (A) Sequence comparison of the zebrafish α1-α6 NC1 domains. Cyan: HSQ tripeptide that begin each NC1 subdomain. Green: 12 conserved cysteine residues. Magenta: additional cysteine (Cysteine 13, Cys-13) in zCol4α4. Red: residues involved in salt bridges formed between the R76 D78, E175-R179, and N187. The red arrow points to the R179K substitution in zCol4α2. Yellow: M93 and K211 residues involved in the peroxidasin-catalyzed sulfilimine bond formation. (B) Representation of the 3D molecular remodeling of human, mouse and zebrafish NC1 domain β-hairpin SM2 subdomain. The additional cysteine (C13) in NC1 β-hairpin of zCol4α4 is indicated by the arrow. The asterisk points to the area where the zebrafish protein backbone is shorter than the human and mouse homologues. (C) Amino acid sequence alignment for human, mouse, and zebrafish NC1 domain, highlighting key residues in the SM2 (hairpin β6–β7) subdomain that indicates less conservation between the α3, α4, and α6 chains compared to the α1, α2, and α5 chains.
Image
Figure Caption
Acknowledgments
This image is the copyrighted work of the attributed author or publisher, and
ZFIN has permission only to display this image to its users.
Additional permissions should be obtained from the applicable author or publisher of the image.
Full text @ Dev. Dyn.