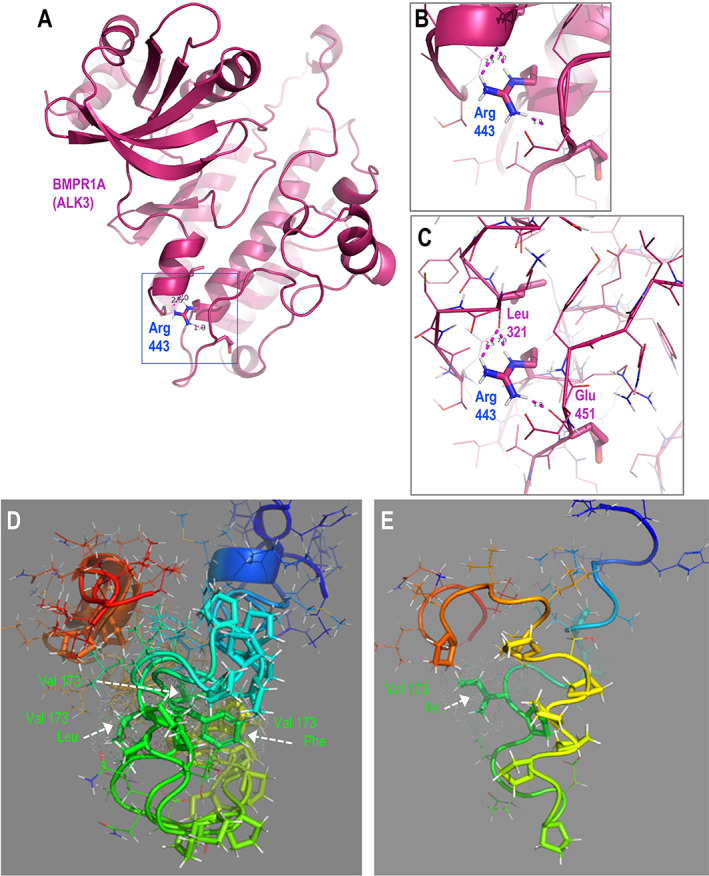
Fig. 2
Structural predictions of BMPR1AR443C and ACVR2AV173I suggest potential functional roles for these protein variants. (A) Structure‐based homology model of the ordered cytoplasmic polypeptide of BMPR1A (regulatory segment and kinase domain). (B) Zoomed view of inset box in A showing the position of the Arg443 residue in the alpha‐helical C‐lobe scaffold, depicted with cartoon representation. (C) View as in B, with the polypeptide backbone represented with ribbon to show the hydrogen‐bonding network between the Arg443 side‐chain hydrogen donors and the backbone acceptors of Leu321 and Glu451. (D,E) De novo predicted structural models for cytoplasmic juxtamembrane segments of ACVR2A wild‐type and V173I forms. (D) Superposition of models for wild‐type (GUU) and putative V173L (CUU), V173F (UUU) segments. (E) Model of V173I (AUU) variant. Note the inversion of the position of the proline‐rich subsegment of the isoleucine variant relative to those in the models for the other bulky hydrophobic (valine, leucine) side‐chains at residue 173 (vertically extended foreground yellow, background green). Wild‐type amino acid sequence: N‐RHHKMAYPPVL V PTQDPGPPPPSPLLGLKPLQLL‐C; residue 173 in bold, underlined; 10 proline residues (10/34) in italics. Polypeptide segments colored in rainbow, N‐terminal blue to C‐terminal red. Side‐chains of residue 173 and the 10 prolines depicted with sticks, all others are lines with exception of the two glycine residues (hydrogens not rendered).