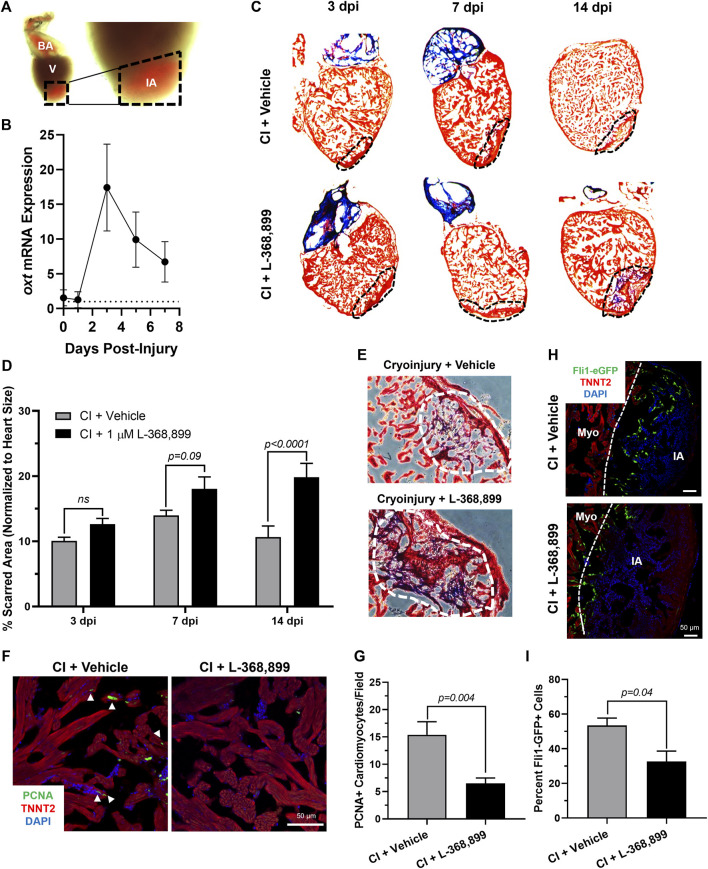
FIGURE 6
Oxytocin Signaling is Necessary for Proper Heart Regeneration in Zebrafish. (A) Example of a freshly cryoinjured zebrafish heart, dashed lines demarcate injured area. (B) Time course qRT-PCR data for oxt in zebrafish brains after cardiac cryoinjury; n = 3 per time point. (C,D) Masson’s trichrome staining (C) and quantification (D) of cryoinjured zebrafish hearts at 3, 7, and 14 dpi treated with and without 1 µM L-368,899. In (C), myocardium is stained red, collagen is stained blue, dashed lines demarcate injured area, which is quantified in (D); n = 3–5 hearts per time point. (E) Representative images of cryoinjured zebrafish myocardium (red) at 14 dpi showing more fibrosis accumulation (blue) after OXTR inhibition. Dashed lines demarcate fibrotic area. (F–G) Confocal immunofluorescent images (F) and quantification (G) of proliferating cardiomyocytes in cryoinjured zebrafish hearts at 3 dpi adjacent to injured areas. In (F), cardiomyocytes are labeled with TNNT2 (red), proliferating cells (arrowheads) with PCNA (green), nuclei with DAPI (blue); n = 8 images per condition, scale bar: 50 µm. (H–I) Confocal immunofluorescent images (H) and quantification (I) of fli1-eGFP transgenic zebrafish at 3 dpi showing endothelial revascularization in cryoinjured and L-368,899-treated hearts. In (H), endothelial and endocardial cells are labeled with GFP (green), cardiomyocytes with TNNT2 (red), nuclei with DAPI (blue); n = 3,4 images per condition, scale bar: 50 μm; BA: Bulbus Arteriosus, CI: Cryoinjury, CM: Cardiomyocyte, dpi: Days Post-Injury, IA: Injured Area, Myo: Myocardium, V: Ventricle.