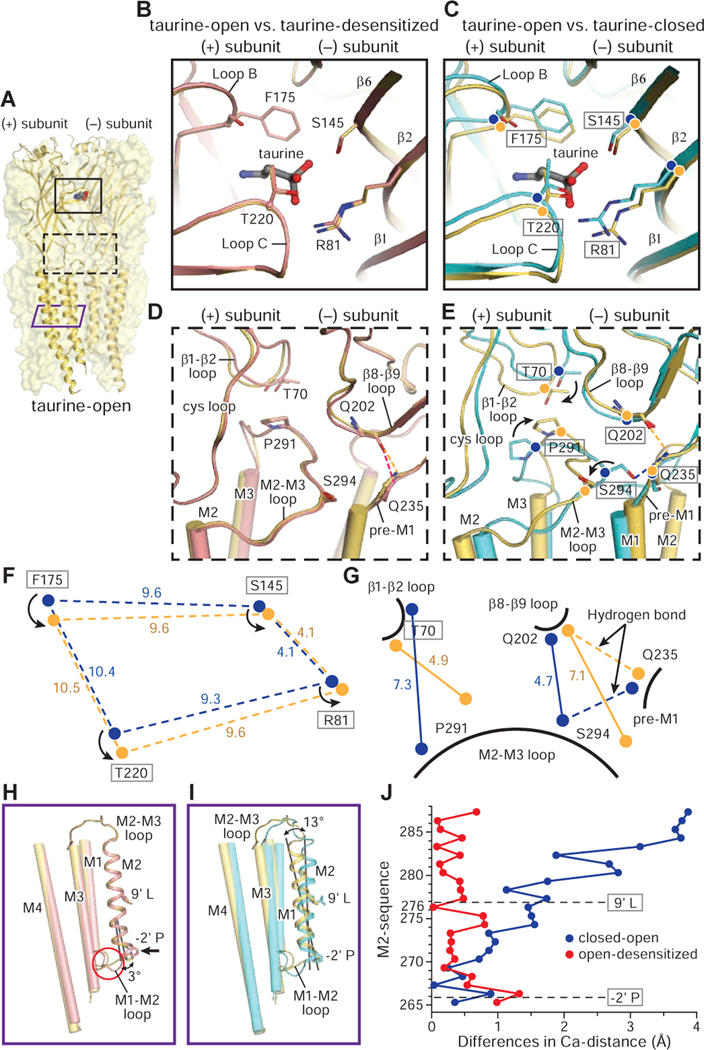
Fig. 6
A. Structure of the taurine-open state. Two subunits are shown in cartoon and the other three subunits are represented as partially transparent surfaces. Taurine is in sphere representation. The binding pocket in the ECD, the ECD-TMD interface and the TMD are indicated by a black solid, a black dashed and a purple solid rectangle, respectively.
B-G. Superposition of the (−) subunit illustrates the relative movements in the (+) subunit. Hydrogen bonds are shown in dashed lines. The taurine-open, taurine-desensitized and taurine-closed states are colored in yellow, salmon and cyan, respectively. (B. D) Comparison of the binding pocket (B) and ECD-TMD interfaces (D) between taurine-open and taurine-desensitized states. (C. E) Conformational changes in the binding pockets (C) and the ECD-TMD interfaces (F) between taurine-closed, taurine-open states. (F. G) Illustration of the changes in distances of the Cα atoms of key residues in the binding pockets (F) and ECD-TMD interfaces (G) between taurine-open and taurine-closed states. Cα atoms in the taurine-open and taurine-closed states are represented by yellow and blue balls, respectively.
H-I. Superimposition of a single subunit illuminates the changes in the TMD between taurine-closed, taurine-open and taurine-desensitized states. The M1, M3 and M4 helices are shown as cylinders while the M2 helix is shown in cartoon representation.
J. The plot of the differences in the position of Cα atoms from residues in the M2 helix derived from the taurine-open, taurine-desensitized and taurine-closed states. Two constriction sites are indicated by dash lines. From open to desensitized states, the major changes are concentrated at the lower half of the M2 helix with −2’P moving by 1.4 Å. By contrast, the M2 helix undergoes a larger conformational change upon transition from the closed to open states.
See also Figures S7–S8.
Reprinted from Cell, 184, Yu, J., Zhu, H., Lape, R., Greiner, T., Du, J., Lü, W., Sivilotti, L., Gouaux, E., Mechanism of gating and partial agonist action in the glycine receptor, 957-968.e21, Copyright (2021) with permission from Elsevier. Full text @ Cell