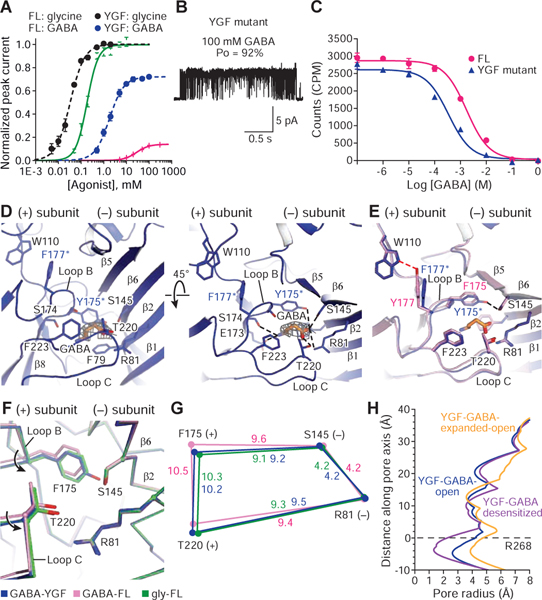
Fig. 4
A. Whole cell, dose response data for glycine and GABA for full length (FL) or the YGF mutant. EC50 for glycine at FL and YGF mutant are 190 ± 20 μM and 33 ± 3 μM, respectively. EC50 for GABA to FL and YGF mutant are 28.4 ± 0.9 mM and 1.05 ± 0.08 mM, respectively. Error bars represent SEM and n≥6 cells for all experiments. Responses are normalized to maximum responses to glycine in each cell.
B. Cell-attached single-channel recording showing openings of the YGF mutant produced by 100 mM GABA.
C. Competition ligand binding experiment using 3H-strychnine. Data are shown as means ± SEM (n=3). Ki of GABA to FL and YGF mutant are 1.41 ± 0.94 mM and 0.18 ± 0.07 mM, respectively. Kd of 3H-strychnine in the FL and YGF mutant are 220 ± 3 nM and 54 ± 4 nM, respectively.
D. GABA binding site in the YGF mutant viewed parallel to the membrane (left) or from the extracellular side of the membrane (right). GABA density is contoured at 0.013 σ. The possible hydrogen bonds and cation-π interactions are shown as dashed lines.
E. Superimposition of the binding pockets from the FL and YGF mutant in the presence of GABA to show the impact on the binding pocket of the swap between Y177 and F175.
F. Conformational changes in the binding pockets of the open states of GABA-bound YGF mutant and FL and glycine-bound FL shown by superposing the ECDs.
G. Schematic diagram illustrating changes in the distances in the binding pocket of the GABA-bound YGF mutant (blue) and FL (pink) as well as glycine-bound FL (green).
H. Plots for pore radius as a function of distance along the pore axis for GABA-bound YGF mutant in the open, desensitized and expanded-open states.
See also Figure S6 and Table S4.
Reprinted from Cell, 184, Yu, J., Zhu, H., Lape, R., Greiner, T., Du, J., Lü, W., Sivilotti, L., Gouaux, E., Mechanism of gating and partial agonist action in the glycine receptor, 957-968.e21, Copyright (2021) with permission from Elsevier. Full text @ Cell