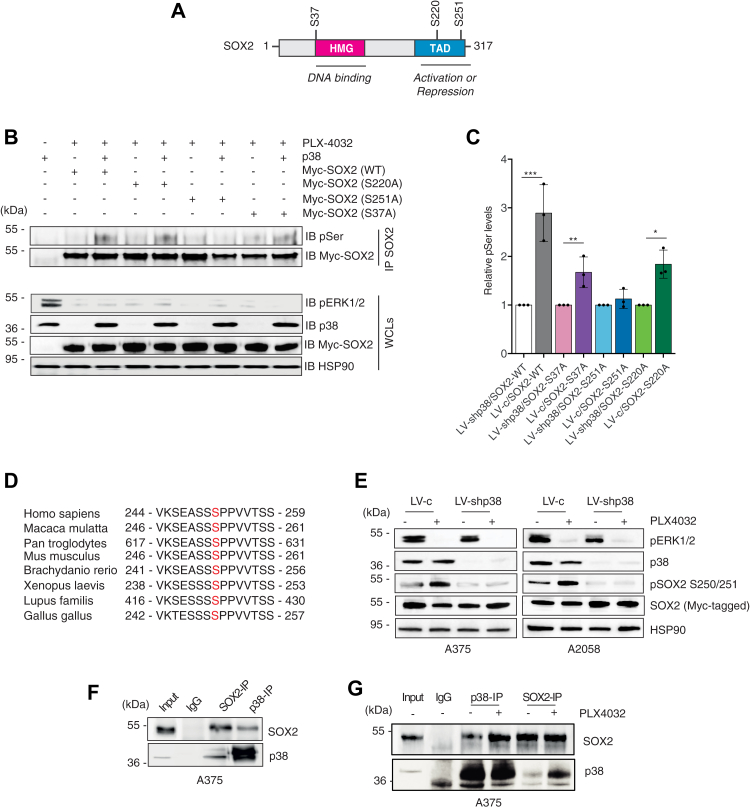
Figure 3
Silencing of p38 abrogates phosphorylation of SOX2 at serine 251.A, schematic representation of SOX2 protein with indicated the high mobility group (HMG), the transactivation domain (TAD), and the three putative p38 phosphorylation sites (S37, S220, S251). B, representative immunoprecipitation of exogenous, Myc-tagged SOX2-WT or mutants (S37A, S220A, S251A) in A375 cells treated PLX-4032 (0.5 μM) for 3 h in presence (LV-c) or absence of p38 (LV-shp38), followed by immunoblotting with antiphospho-serine (pSer) antibody, in presence (LV-c) or absence of p38 (LV-shp38). C, quantification of pSer after IP of SOX2, expressed as mean ± SD of three independent experiments, with the level induced by SOX2 WT, SOX2-S37A, SOX2-S220A, and SOX2-S251A in absence of p38 equated to 1. p value was calculated by two-tailed unpaired Student’s t test. Note that p38 increases phosphorylation of SOX2-WT, SOX2-S37A, and SOX2-S220A but not of SOX2-S251A. D, residue Ser251 lies in a region of human SOX2 proteins conserved among species. E, representative Western blot of pERK1/2, p38α/β, pSOX2-S250/S251, and SOX2 in A375 and A2058 cells transduced with LV-c or LV-shp38, transiently transfected with exogenous, Myc-tagged SOX2 and treated with DMSO or PLX-4032 (0.5 μM) for 3 h. HSP90 was used as loading control. F, coimmunoprecipitation (co-IP) of SOX2 and p38 in A375 lysates. Input was 5%. G, co-IP of SOX2 and p38 in A375 lysates upon treatment with DMSO or PLX-4032 (0.5 μM) for 3 h. Input was 5%. Molecular weight markers are noted next to all immunoblots. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001. DMSO, dimethyl sulfoxide.