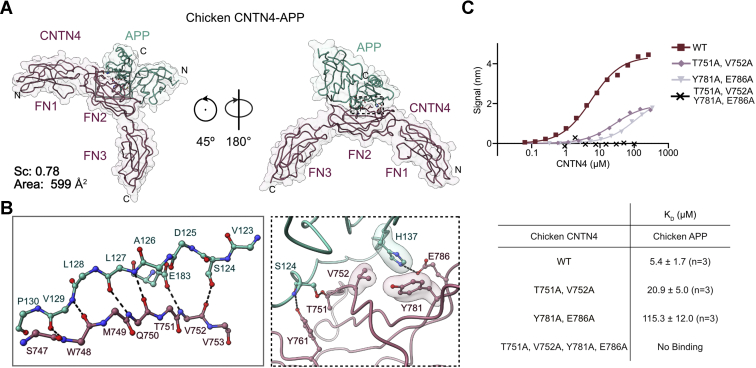
Figure 4
Structural basis for interactions between APP and CNTN4.A, the crystal structure of chicken APP(E1) (green) bound to CNTN4(FN1–FN3) (purple) is shown in a coil representation superimposed with a translucent surface. The letters N and C indicate the N and C termini, respectively. The interface area occludes 599 Å2 of surface area, whereas the shape complementarity (Sc) statistic is consistent with protease–inhibitor complexes (39). B, detailed views of the interfaces boxed in A showing hydrogen bonding interactions between antiparallel β-strands in CNTN4 and APP. Most contacts involve main-chain hydrogen bonding interactions although the side-chain carboxylate group of E183 and the hydroxyl group of S124 form hydrogen bonds with the nitrogen and oxygen atoms of V752 in CNTN4, respectively. Noninteracting side-chain atoms are not shown for clarity. Dashed lines indicate potential hydrogen bonds or salt bridges, whereas translucent surfaces highlight residues involved in packing interactions. C, analysis of the chicken CNTN4–APP interface by site-directed mutagenesis. The biotinylated E1 domain of chicken APP was immobilized onto streptavidin sensors and titrated against varying concentrations of the FN1–FN3 region of chicken CNTN4 proteins. One representative experiment for each series is shown in the top panel, whereas a summary of the affinities measured for at least three biological replicates is shown below the binding curves. APP, amyloid precursor protein; CNTN4, contactin 4; FN1, first FN repeat; FN3, third FN repeat.