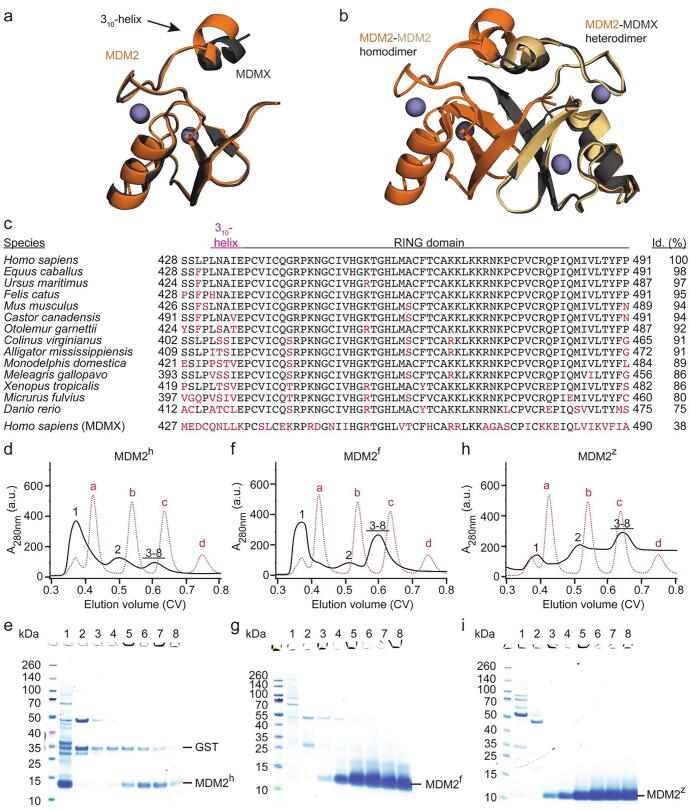
Figure 1
Aggregation of the MDM2 RING domain is species dependent. (a) Superimposition of human MDM2 428-C (orange) and MDMX 428-C (dark-gray) (PDB: 5MNJ). Zinc ions are shown as gray spheres. The 310-helix preceding the RING domain is indicated. (b) Superimposition of the MDM2 RING domain homodimer (PDB: 6SQO; the two monomeric MDM2 RING domains are colored in orange and light orange) and human MDM2-MDMX RING domain heterodimer (PDB: 5MNJ; MDM2 is orange and MDMX is dark-gray). Zinc ions are shown as gray spheres. (c) Sequence alignment of the C-terminal region of MDM2 from different species. The regions corresponding to the 310-helix in MDM2h and the RING domain are indicated. Sequence deviations from human MDM2 are highlighted in red. The sequence identity (id.) compared to human MDM2 is indicated on the right. (d, f, h) Superdex 75 elution profiles of MDM2h, MDM2f and MDM2z, respectively (shown in black solid line). The elution profile of molecular weight markers (a: bovine serum albumin, 66 kDa; b: carbonic anhydrase, 29 kDa; c: cytochrome C, 12.4 kDa; d: aprotinin, 6,5 kDa) is shown as red dashed line. After removal of GST-tag, the cleaved MDM2 variants (expressed from 24L LB) were applied on a HiLoad 16/600 Superdex 75 column. (e, g, i) SDS-PAGE of indicated fractions from panels d, f, h, respectively. The position of the fractions within the elution profile is indicated by numbers (1–8).