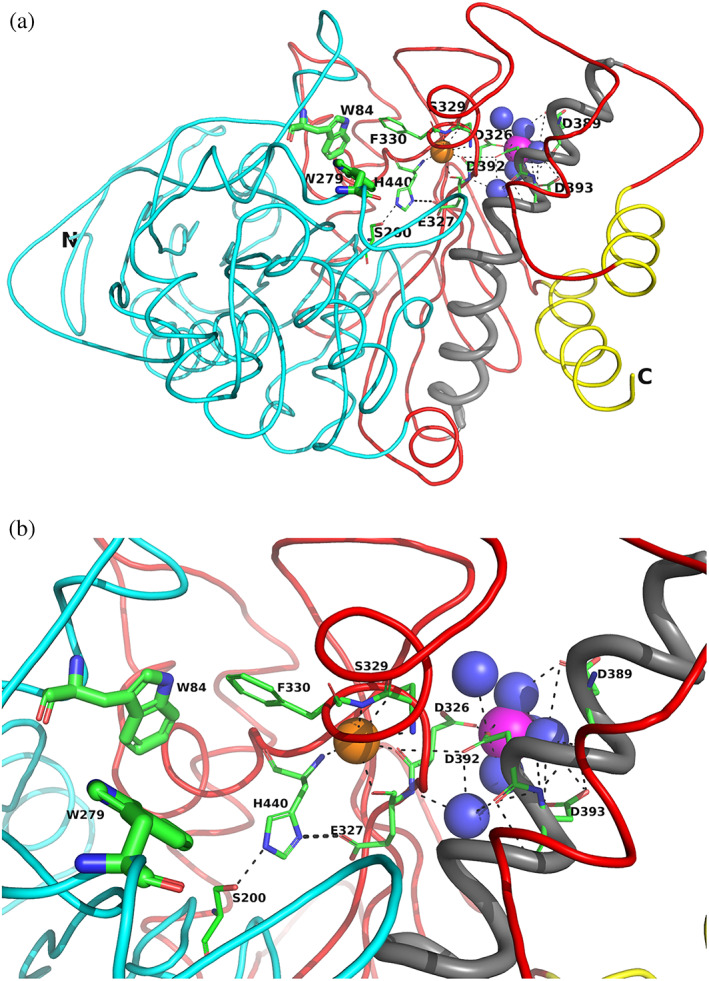
FIGURE 6 Overall views of the Mg+2/TcAChE complex. (a) Ribbon diagram of the Mg+2/TcAChE complex. The representation shows the entire structure, with the first subdomain, residues 4–305, in cyan, and the second, residues 306–535, in red. It is oriented looking into the active‐site gorge, with W279, in the peripheral anionic site (PAS), at the top of the gorge, and W84, in the catalytic anionic site (CAS) toward the back, adjacent to the catalytic triad, S200‐E327‐H440. All these residues are depicted as sticks. The long α‐helix, N383‐K413, against which the 4D pocket is glued, is in grey, and the two helices that contribute to the four‐helix bundle of the dimer, D365‐Y375 and V518‐T535, are in yellow. The Mg+2 in the 4D pocket is in magenta, and is surrounded by four waters in blue. A conserved water H‐bonds with D326, of the 4D motif, with E327 and H440, in the catalytic triad, and with the main‐chain nitrogen of F330, which, in turn, contributes to the CAS. This water which is homologous to water 623 in Koellner et al.,29 is shown as an orange sphere. (b) Close up, with the same orientation, showing the interactions of the active site, the 4D pocket, and the conserved water, shown as an orange sphere
Image
Figure Caption
Acknowledgments
This image is the copyrighted work of the attributed author or publisher, and
ZFIN has permission only to display this image to its users.
Additional permissions should be obtained from the applicable author or publisher of the image.
Full text @ Protein Sci.