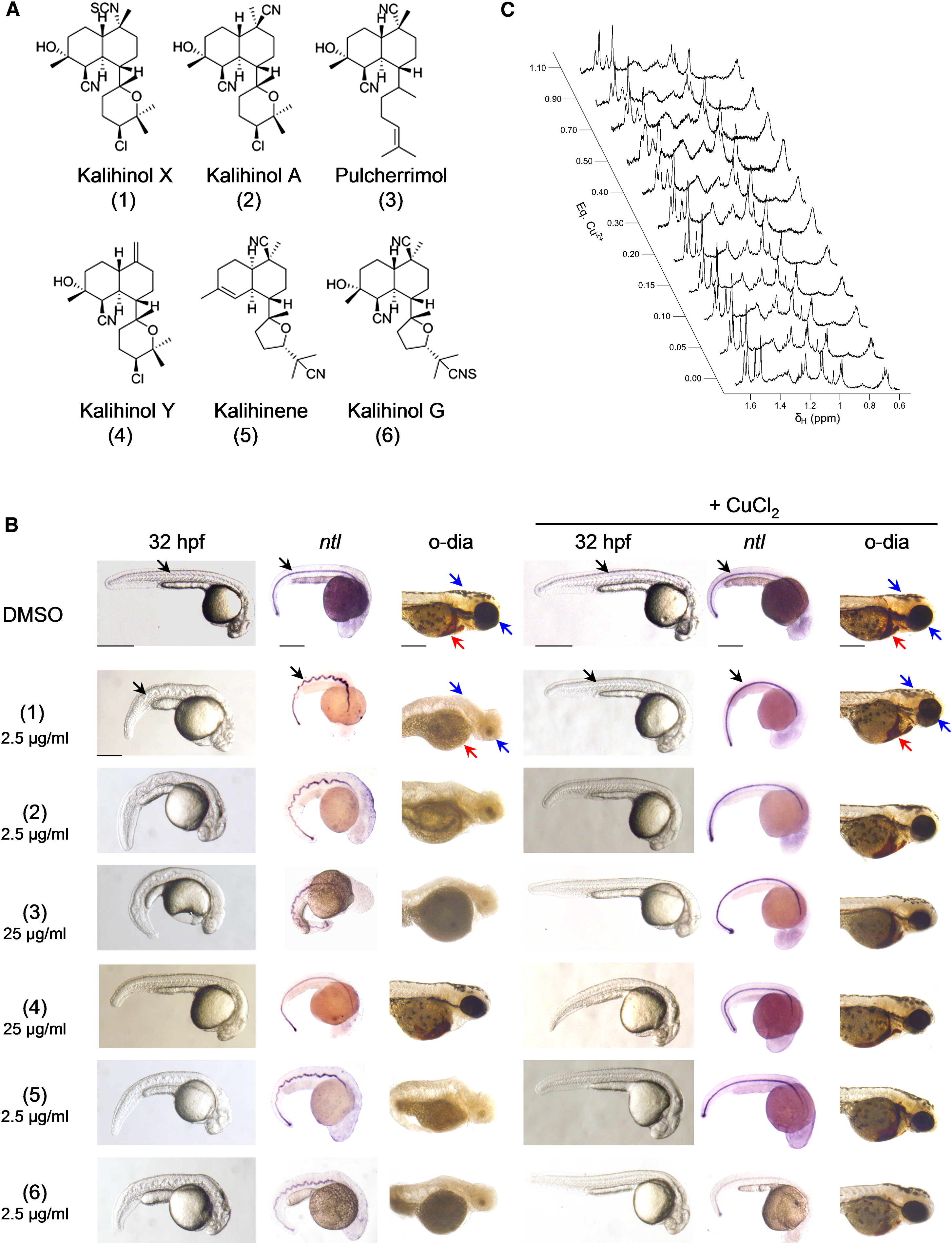
Fig. 5 Other Members of the Kalihinol Family also Show Copper Chelating Activity (A) Chemical structure of kalihinol analogs. (B) Seven hr postfertilization embryos were treated with either 25 μg/ml or 2.5 μg/ml of kalihinol analogs and DMSO vehicle control (first, second, and third columns). For phenotype rescue experiments, 5 hpf embryos were treated with kalihinols and given CuCl2 at 7 hpf (fourth, fifth, and sixth columns). Embryos were monitored for wavy notochord (black arrow), loss of pigmentation (blue arrow), and hematopoiesis (red arrow).(C) Effects of addition of Cu2+ to the 1H NMR line widths of the methyl signal region of pulcherrimol.
Image
Figure Caption
Acknowledgments
This image is the copyrighted work of the attributed author or publisher, and
ZFIN has permission only to display this image to its users.
Additional permissions should be obtained from the applicable author or publisher of the image.
Full text @ Chem. Biol.