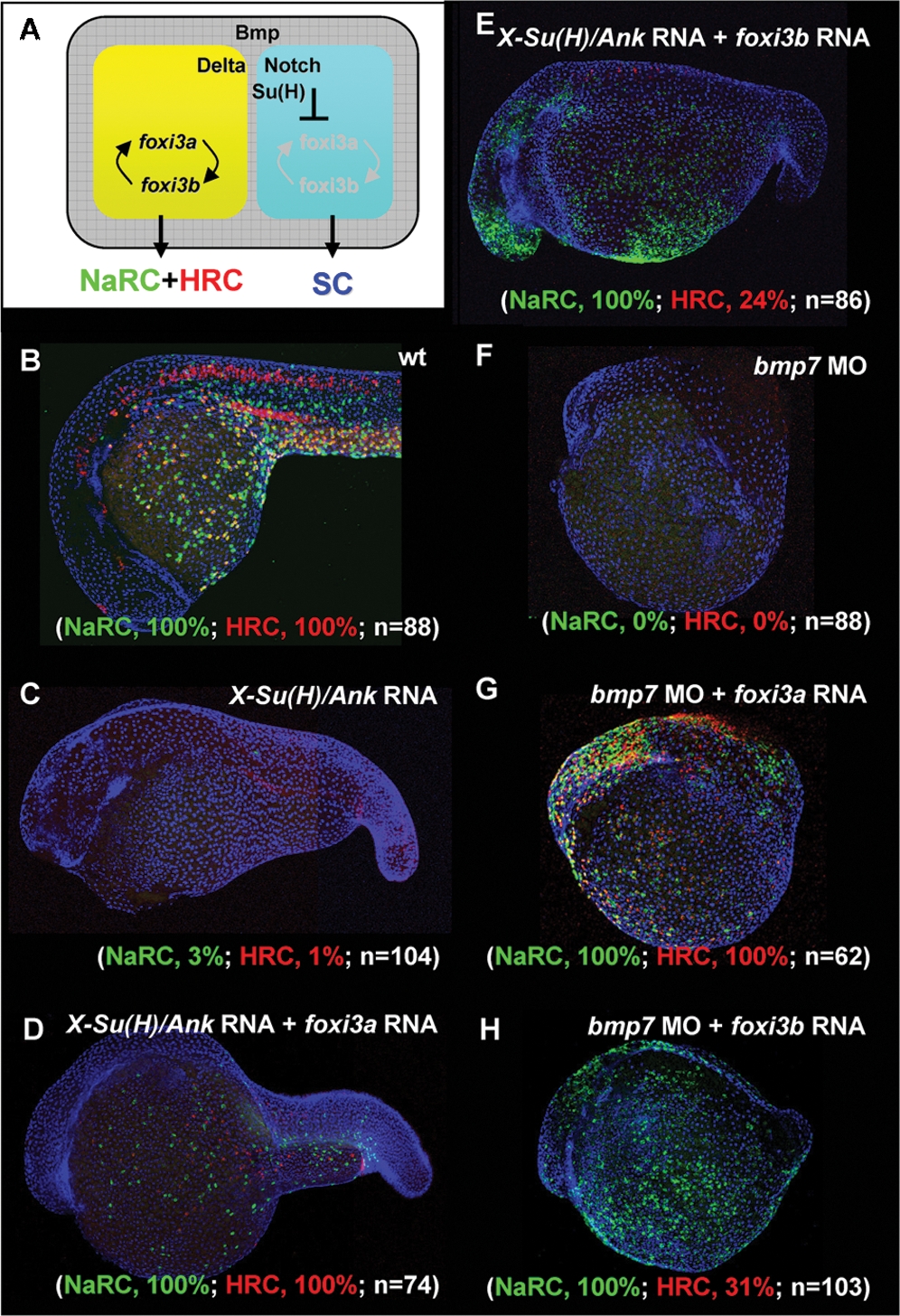
Fig. 8 Dissection of the Function of foxi3a and foxi3b in X-Su(H)/Ank mRNA-Injected Embryos or bmp7 Morphants. (A) Schematic diagram showing the role of Bmp and Delta-Notch signaling in specifying the epidermal ectoderm (gray) and singling-out epidermal ionocytes (yellow) from the epidermal stem cell pool (blue). (B) Normal Na+,K+-ATPase rich cell (NaRC) and H+-ATPase rich cell (HRC) differentiation in wild-type embryos aged at 24 hours post-fertilization (hpf). In wild-type embryos, a few IC progenitors were selected from the epidermal stem cell pool within the epidermal ionocyte domain by Delta-Notch-mediated lateral inhibition, and then differentiated into NaRCs (green, detected by atp1b1b in situ) or HRCs (red, detected by ca2a in situ) which are scattered on the epidermal layer. (C) For X-Su(H)/Ank mRNA-injected embryos (500 pg/embryo), the foxi3a and foxi3b expressions were inhibited due to the elevated Notch activity in the epidermal ionocyte domain. In such a condition, all epidermal ionocyte progenitors adopted the epidermal stem cell fate; therefore, subsequent NaRC and HRC differentiation was completely abolished. (D) When foxi3a mRNA (50 pg/embryo) and X-Su(H)/Ank mRNA (500 pg/embryo) were co-injected, high levels of exogenous foxi3a expression could compensate for the elevated Notch activity and restore both NaRC and HRC differentiation. (E) When foxi3b mRNA (50 pg/embryo) and X-Su(H)/Ank mRNA (500 pg/embryo) were co-injected, a high level of exogenous foxi3b expression was also sufficient to compensate for the elevated Notch activity to restore NaRC and partially restore HRC differentiation. (F) In bmp7 morphants (0.1 mM/embryo), although the low level of Bmp signals was still sufficient to promote P63 expression, it was insufficient to drive either foxi3a or foxi3b expression and finally the epidermal ionocytes lost their identity. (G) When foxi3a mRNA (50 pg/embryo) and bmp7 MO (0.1 mM/embryo) were co-injected, a high level of exogenous foxi3a expression could compensate for the low Bmp activity and restore both NaRC and HRC differentiation. (H) When foxi3b mRNA (50 pg/embryo) and bmp7 MO (0.1 mM/embryo) were co-injected, a high level of exogenous foxi3b expression could compensate for the low Bmp activity to restore NaRC and partial HRC differentiation. All embryos were scored at 24 hpf.
Image
Figure Caption
Figure Data
Acknowledgments
This image is the copyrighted work of the attributed author or publisher, and
ZFIN has permission only to display this image to its users.
Additional permissions should be obtained from the applicable author or publisher of the image.
Full text @ PLoS One