Fig. 1
- ID
- ZDB-FIG-240528-13
- Publication
- Wang et al., 2023 - Stability and function of RCL1 are dependent on interaction with BMS1
- Other Figures
- All Figure Page
- Back to All Figure Page
|
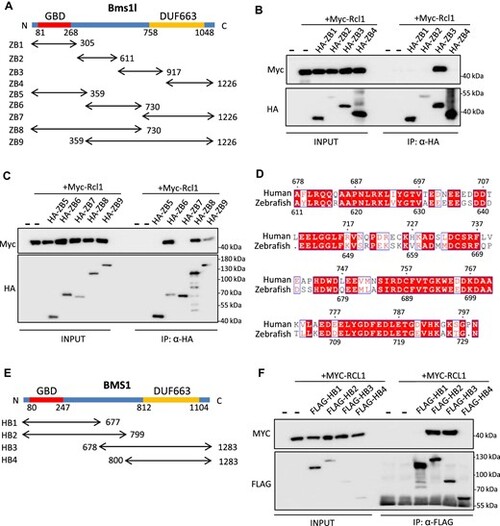
RCL1-interacting domain in BMS1 is conserved in zebrafish and humans. (A) Diagram illustrating zebrafish ZB1–ZB9. ZB1–ZB4 were divided evenly and ZB5–ZB9 were divided based on domain structure by sequence alignment with yeast Bms1. GBD, GTP-binding domain; DUF663, protein of unknown function. (B and C) Co-IP analysis of interactions between Myc-Rcl1 and HA-tagged ZB1–ZB4 (B) and ZB5–ZB9 (C). HA beads were used to pull down HA-tagged ZB fragments. ZB3, ZB6, ZB8, and ZB9 interacted with Rcl1. (D) Amino acid sequence alignment between 611–730 aa region of zebrafish Bms1l and 678–799 aa region of human BMS1. Red boxes represent identical amino acids. (E) Diagram illustrating human HB1–HB4 based on predicted interacting domain. (F) Co-IP analysis of interactions between MYC-RCL1 and FLAG-tagged HB1–HB4. Flag beads were used to pull down FLAG-tagged HB fragments. Both HB2 and HB3 interacted with RCL1. |