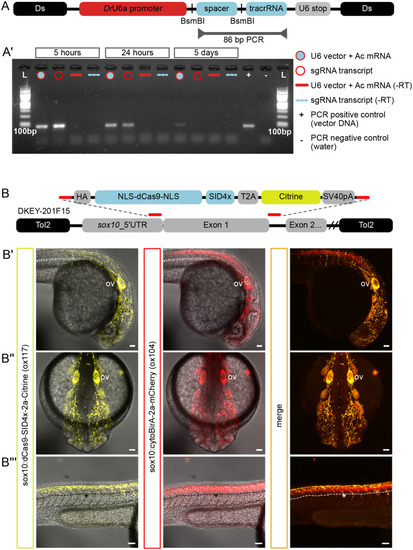
Components of a CRISPRi genetic toolkit in zebrafish. (A) Schematic of Ac/Ds construct containing a guide RNA region (‘spacer’ and ‘tracrRNA’) cloned downstream of the zebrafish U6a promoter (‘DrU6a promoter’) followed by a U6 polymerase termination sequence (‘U6 stop’). The ‘spacer’ region is flanked with BsmBI restriction sites for Golden-Gate-like cloning of target spacers-of-choice. To evaluate expression of a scrambled guide RNA, RT-PCR primers and conditions were optimised to produce an 86 bp amplicon spanning the spacer and tracrRNA. (A′) RNA was collected from embryos injected with either the U6 vector scrambled sgRNA (filled circle) or in vitro-transcribed sgRNAs (empty circle) at 5 h, 24 h and 5 days post-injection. Only the RT-PCR product from U6-sgRNA injections could be detected at 5 days. (B) Schematic of BAC recombination to generate a Sox10-specific CRISPRi transgenic line, TgBAC(sox10:dCas9-SID4x-2a-Citrine)ox117. A recombination cassette contains nuclease-deficient Cas9 (‘NLS-dCas9-NLS’) fused to the SID4x repressor domain (‘SID4x’) followed by a ribosome-skipping Tav-2a peptide (‘T2A’) and Citrine fluorescent protein (‘Citrine’). Homology arms (red lines) enabled replacement of sox10’s first exon in BAC clone DKEY-201F15 with the dCas9-SID4x cassette. Transgenic offspring displayed largely overlapping expression with a different allele made from the same BAC (TgBAC(sox10:cytoBirA-2a-mCherry), ‘ox104’) in cranial and trunk neural crest cells, as well as the otic vesicle (ov) (B′,B″). However, unlike the ox104 line, Citrine is not expressed in ox117’s neural tube (B‴). Scale bars: 50 µm.
|