|
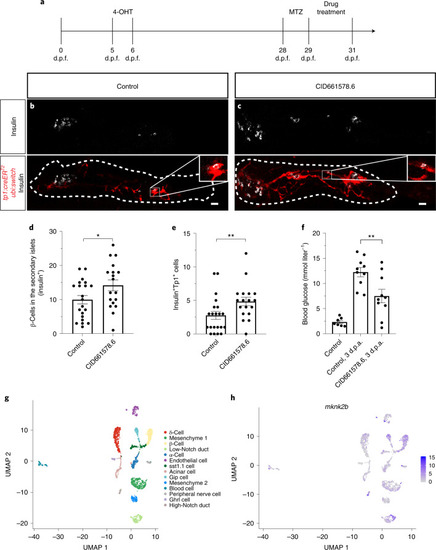
CID661578.6 increases β-cell regeneration from a pancreatic ductal origin and lowers glucose levels.a, Schematic of the lineage tracing experiment. Briefly, larvae were treated with 4-hydroxytamoxifen (4-OHT) for 24 h (5-6 d.p.f.) to induce recombination of the reporter. At 28 d.p.f., the fish were treated with MTZ for 24 h to ablate the β-cells, followed by 48 h of treatment with DMSO or CID661578.6. b–e, Representative images of Tg(ubi:switch); Tg(tp1:creERT2); Tg(ins:flag-NTR) fish treated with DMSO (b) or 2 µM CID661578.6 (c) and immunostained for insulin at 31 d.p.f.; scale bars, 20 µm. Quantifications of the number of β-cells in the secondary islets along the tail of the pancreas (d) as well as the number of β-cells derived from Notch-responsive cells (e) are shown; n = 21 (control) and n = 18 (CID661578.6) for d–e. An unpaired two-tailed Student’s t-test was used to assess significance for d (*P = 0.0393), and a two-tailed Mann–Whitney test was used for e (**P = 0.0087). Data are presented as mean values ± s.e.m. The experiment shown in b and c was repeated twice with similar results. f, Blood glucose was measured 3 d post-β-cell ablation (d.p.a.) in 4-month-old fish treated with DMSO or CID661578.6. Blood glucose levels in zebrafish without β-cell ablation were included as a basal-state reference; n = 7 (control), n = 10 (control, 3 d.p.a.), n = 10 (CID661578.6, 3 d.p.a.). A one-way ANOVA followed by Šidák’s multiple comparisons test was used to assess significance for f (**P = 0.0078). Data are presented as mean values ± s.e.m. g,h, UMAP plots showing the different cell types present in the adult zebrafish pancreas after reanalysis of published single-cell RNA-seq data (g) and expression of mknk2b (h) at various levels in the different clusters. Source data
|